A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast
- PMID: 19194512
- PMCID: PMC2633045
- DOI: 10.1371/journal.pone.0004376
A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast
Abstract
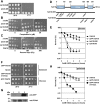
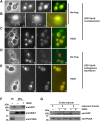
A mutated allele of the essential gene TAH18 was previously identified in our laboratory in a genetic screen for new proteins interacting with the DNA polymerase delta in yeast [1]. The present work shows that Tah18 plays a role in response to oxidative stress. After exposure to lethal doses of H(2)O(2), GFP-Tah18 relocalizes to the mitochondria and controls mitochondria integrity and cell death. Dre2, an essential Fe/S cluster protein and homologue of human anti-apoptotic Ciapin1, was identified as a molecular partner of Tah18 in the absence of stress. Moreover, Ciapin1 is able to replace yeast Dre2 in vivo and physically interacts with Tah18. Our results are in favour of an oxidative stress-induced cell death in yeast that involves mitochondria and is controlled by the newly identified Dre2-Tah18 complex.
Conflict of interest statement
Figures



Similar articles
-
Interaction between the reductase Tah18 and highly conserved Fe-S containing Dre2 C-terminus is essential for yeast viability.Mol Microbiol. 2011 Oct;82(1):54-67. doi: 10.1111/j.1365-2958.2011.07788.x. Epub 2011 Sep 8. Mol Microbiol. 2011. PMID: 21902732
-
Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis.Nat Chem Biol. 2010 Oct;6(10):758-65. doi: 10.1038/nchembio.432. Epub 2010 Aug 29. Nat Chem Biol. 2010. PMID: 20802492
-
Regulatory mechanism of the flavoprotein Tah18-dependent nitric oxide synthesis and cell death in yeast.Nitric Oxide. 2016 Jul 1;57:85-91. doi: 10.1016/j.niox.2016.04.003. Epub 2016 May 10. Nitric Oxide. 2016. PMID: 27178802
-
Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis.Mol Cell Biol. 2008 Sep;28(18):5569-82. doi: 10.1128/MCB.00642-08. Epub 2008 Jul 14. Mol Cell Biol. 2008. PMID: 18625724 Free PMC article.
-
Fe-S Cluster Hsp70 Chaperones: The ATPase Cycle and Protein Interactions.Methods Enzymol. 2017;595:161-184. doi: 10.1016/bs.mie.2017.07.004. Epub 2017 Aug 21. Methods Enzymol. 2017. PMID: 28882200 Free PMC article. Review.
Cited by
-
Biogenesis of Iron-Sulfur Clusters and Their Role in DNA Metabolism.Front Cell Dev Biol. 2021 Sep 30;9:735678. doi: 10.3389/fcell.2021.735678. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34660592 Free PMC article. Review.
-
Conserved electron donor complex Dre2-Tah18 is required for ribonucleotide reductase metallocofactor assembly and DNA synthesis.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):E1695-704. doi: 10.1073/pnas.1405204111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733891 Free PMC article.
-
Yeast AP-1 like transcription factors (Yap) and stress response: a current overview.Microb Cell. 2019 May 28;6(6):267-285. doi: 10.15698/mic2019.06.679. Microb Cell. 2019. PMID: 31172012 Free PMC article. Review.
-
Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism-Review.Molecules. 2020 Aug 25;25(17):3860. doi: 10.3390/molecules25173860. Molecules. 2020. PMID: 32854270 Free PMC article. Review.
-
Anamorsin, a novel caspase-3 substrate in neurodegeneration.J Biol Chem. 2014 Aug 8;289(32):22183-95. doi: 10.1074/jbc.M114.552679. Epub 2014 Jun 27. J Biol Chem. 2014. PMID: 24973211 Free PMC article.
References
-
- Chanet R, Heude M. Characterization of mutations that are synthetic lethal with pol3-13, a mutated allele of DNA polymerase delta in Saccharomyces cerevisiae. Curr Genet. 2003;43:337–350. - PubMed
-
- Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

