Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity
- PMID: 19194884
- PMCID: PMC2665303
- DOI: 10.1002/ana.21526
Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity
Abstract
Objective: Although vigabatrin irreversibly constricts the visual field, it remains a potent therapy for infantile spasms and a third-line drug for refractory epilepsies. In albino animals, this drug induces a reduction in retinal cell function, retinal disorganization, and cone photoreceptor damage. The objective of this study was to investigate the light dependence of the vigabatrin-elicited retinal toxicity and to screen for molecules preventing this secondary effect of vigabatrin.
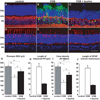
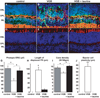
Methods: Rats and mice were treated daily with 40 and 3mg vigabatrin, respectively. Retinal cell lesions were demonstrated by assessing cell function with electroretinogram measurements, and quantifying retinal disorganization, gliosis, and cone cell densities.
Results: Vigabatrin-elicited retinal lesions were prevented by maintaining animals in darkness during treatment. Different mechanisms including taurine deficiency were reported to produce such phototoxicity; we therefore measured amino acid plasma levels in vigabatrin-treated animals. Taurine levels were 67% lower in vigabatrin-treated animals than in control animals. Taurine supplementation reduced all components of retinal lesions in both rats and mice. Among six vigabatrin-treated infants, the taurine plasma level was found to be below normal in three patients and undetectable in two patients.
Interpretation: These results indicate that vigabatrin generates a taurine deficiency responsible for its retinal phototoxicity. Future studies will investigate whether cotreatment with taurine and vigabatrin can limit epileptic seizures without inducing the constriction of the visual field. Patients taking vigabatrin could gain immediate benefit from reduced light exposures and dietetic advice on taurine-rich foods.
Conflict of interest statement
Figures


References
-
- Ben-Menachem E, Dulac O, Chiron C. Vigabatrin. In: Engel Jerome, Jr, Pedley Timothy A., editors. Epilepsy: a comprehensive text book. Second edition. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 1683–1693.
-
- Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–618. - PubMed
-
- Ruether K, Pung T, Kellner U, et al. Electrophysiologic evaluation of a patient with peripheral visual field contraction associated with vigabatrin. Archives of ophthalmology. 1998;116:817–819. - PubMed
-
- Johnson MA, Krauss GL, Miller NR, et al. Visual function loss from vigabatrin: effect of stopping the drug. Neurology. 2000;55:40–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

