A mutant hepatitis B virus core protein mimics inhibitors of icosahedral capsid self-assembly
- PMID: 19196007
- PMCID: PMC2880625
- DOI: 10.1021/bi801814y
A mutant hepatitis B virus core protein mimics inhibitors of icosahedral capsid self-assembly
Abstract
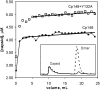
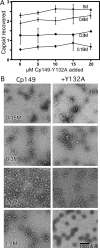
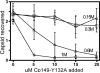
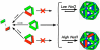
Understanding self-assembly of icosahedral virus capsids is critical to developing assembly directed antiviral approaches and will also contribute to the development of self-assembling nanostructures. One approach to controlling assembly would be through the use of assembly inhibitors. Here we use Cp149, the assembly domain of the hepatitis B virus capsid protein, together with an assembly defective mutant, Cp149-Y132A, to examine the limits of the efficacy of assembly inhibitors. By itself, Cp149-Y132A will not form capsids. However, Cp-Y132A will coassemble with the wild-type protein on the basis of light scattering and size exclusion chromatography. The resulting capsids appear to be indistinguishable from normal capsids. However, coassembled capsids are more fragile, with disassembly observed by chromatography under mildly destabilizing conditions. The relative persistence of capsids assembled under conditions where association energy is weak compared to the fragility of those where association is strong suggests a mechanism of "thermodynamic editing" that allows replacement of defective proteins in a weakly associated complex. There is fine line between weak assembly, where assembly defective protein is edited from a growing capsid, and relatively strong assembly, where assembly defective subunits may dramatically compromise virus stability. Thus, attempts to control virus self-assembly (with small molecules or defective proteins) must take into account the competing process of thermodynamic editing.
Figures



References
-
- Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Straus SE. Virology. 3 ed. Lippincott-Raven Publishers; Philadelphia: 1996.
-
- Douglas T, Young M. Nature. 1998;393:152–155.
-
- Douglas T, Young M. Science. 2006;312:873–5. - PubMed
-
- Wang Q, Lin T, Tang L, Johnson JE, Finn MG. Angew Chem Int Ed Engl. 2002;41:459–62. - PubMed
-
- Falkner JC, Turner ME, Bosworth JK, Trentler TJ, Johnson JE, Lin T, Colvin VL. J Am Chem Soc. 2005;127:5274–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

