Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture
- PMID: 19196140
- PMCID: PMC2811059
- DOI: 10.1089/ten.tea.2008.0455
Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture
Abstract
Embryonic stem cells (ESCs) with their abilities for extensive proliferation and multi-lineage differentiation can serve as a renewable source of cellular material in regenerative medicine. However, the development of processes for large-scale generation of human ESCs (hESCs) or their progeny will be necessary before hESC-based therapies become a reality. We hypothesized that microcarrier stirred-suspension bioreactors characterized by scalability, straightforward operation, and tight control of the culture environment can be used for hESC culture and directed differentiation. Under appropriate conditions, the concentration of hESCs cultured in a microcarrier bioreactor increased 34- to 45-fold over 8 days. The cells retained the expression of pluripotency markers such as OCT3/4A, NANOG, and SSEA4, as assessed by quantitative PCR, immunocytochemistry, and flow cytometry. We further hypothesized that hESCs on microcarriers can be induced to definitive endoderm (DE) when incubated with physiologically relevant factors. In contrast to embryoid body cultures, all hESCs on microcarriers are exposed to soluble stimuli in the bulk medium facilitating efficient transition to DE. After reaching a peak concentration, hESCs in microcarrier cultures were incubated in medium containing activin A, Wnt3a, and low concentration of serum. More than 80% of differentiated hESCs coexpressed FOXA2 and SOX17 in addition to other DE markers, whereas the expression of non-DE genes was either absent or minimal. We also demonstrate that the hESC-to-DE induction in microcarrier cultures is scalable. Our findings support the use of microcarrier bioreactors for the generation of endoderm progeny from hESCs including pancreatic islets and liver cells in therapeutically useful quantities.
Figures

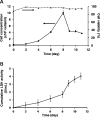
 ), 10 × 104 (
), 10 × 104 ( ), and 20 × 104 (
), and 20 × 104 ( ) hESCs/mL and cultured at 45 rpm. (B) Human ESCs were seeded at 1 × 105 cells/mL and propagated at 45 (
) hESCs/mL and cultured at 45 rpm. (B) Human ESCs were seeded at 1 × 105 cells/mL and propagated at 45 ( ), 60 (
), 60 ( ), and 80 (
), and 80 ( ) rpm. (C) Colonization of beads by hESCs cultured at 60 rpm. (D) FDA/PI staining of hESCs on beads at 45 rpm (day 8). (E) Cumulative LDH activity for hESCs cultured at 45 rpm. Bars in (D): 50 μm. Color images available online at
) rpm. (C) Colonization of beads by hESCs cultured at 60 rpm. (D) FDA/PI staining of hESCs on beads at 45 rpm (day 8). (E) Cumulative LDH activity for hESCs cultured at 45 rpm. Bars in (D): 50 μm. Color images available online at 
 ), 10 × 104 (
), 10 × 104 ( ), and 20 × 104 (
), and 20 × 104 ( ) hESCs/mL and cultured at 45 rpm. (B) Human ESCs were seeded at 1 × 105 cells/mL and propagated at 45 (
) hESCs/mL and cultured at 45 rpm. (B) Human ESCs were seeded at 1 × 105 cells/mL and propagated at 45 ( ), 60 (
), 60 ( ), and 80 (
), and 80 ( ) rpm. (C) Colonization of beads by hESCs cultured at 60 rpm. (D) FDA/PI staining of hESCs on beads at 45 rpm (day 8). (E) Cumulative LDH activity for hESCs cultured at 45 rpm. Bars in (D): 50 μm. Color images available online at
) rpm. (C) Colonization of beads by hESCs cultured at 60 rpm. (D) FDA/PI staining of hESCs on beads at 45 rpm (day 8). (E) Cumulative LDH activity for hESCs cultured at 45 rpm. Bars in (D): 50 μm. Color images available online at 






References
-
- Ryan E.A. Paty B.W. Senior P.A. Bigam D. Alfadhli E. Kneteman N.M. Lakey J.R. Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. - PubMed
-
- D'Amour K.A. Bang A.G. Eliazer S. Kelly O.G. Agulnick A.D. Smart N.G. Moorman M.A. Kroon E. Carpenter M.K. Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392. - PubMed
-
- Jiang J. Au M. Lu K. Eshpeter A. Korbutt G. Fisk G. Majumdar A.S. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940. - PubMed
-
- Kroon E. Martinson L.A. Kadoya K. Bang A.G. Kelly O.G. Eliazer S. Young H. Richardson M. Smart N.G. Cunningham J. Agulnick A.D. D'Amour K.A. Carpenter M.K. Baetge E.E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443. - PubMed
-
- Fong W.J. Tan H.L. Choo A. Oh S.K. Perfusion cultures of human embryonic stem cells. Bioprocess Biosyst Eng. 2005;27:381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

