AMPK and the biochemistry of exercise: implications for human health and disease
- PMID: 19196246
- PMCID: PMC2779044
- DOI: 10.1042/BJ20082055
AMPK and the biochemistry of exercise: implications for human health and disease
Abstract
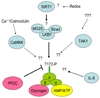
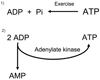
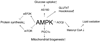
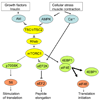
AMPK (AMP-activated protein kinase) is a phylogenetically conserved fuel-sensing enzyme that is present in all mammalian cells. During exercise, it is activated in skeletal muscle in humans, and at least in rodents, also in adipose tissue, liver and perhaps other organs by events that increase the AMP/ATP ratio. When activated, AMPK stimulates energy-generating processes such as glucose uptake and fatty acid oxidation and decreases energy-consuming processes such as protein and lipid synthesis. Exercise is perhaps the most powerful physiological activator of AMPK and a unique model for studying its many physiological roles. In addition, it improves the metabolic status of rodents with a metabolic syndrome phenotype, as does treatment with AMPK-activating agents; it is therefore tempting to attribute the therapeutic benefits of regular physical activity to activation of AMPK. Here we review the acute and chronic effects of exercise on AMPK activity in skeletal muscle and other tissues. We also discuss the potential role of AMPK activation in mediating the prevention and treatment by exercise of specific disorders associated with the metabolic syndrome, including Type 2 diabetes and Alzheimer's disease.
Figures
References
-
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J.Biol.Chem. 2004;279:12005–12008. - PubMed
-
- Andersson U, Treebak JT, Nielsen JN, Smith KL, Abbott CR, Small CJ, Carling D, Richter EA. Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity. Biochem.Biophys.Res.Commun. 2005;329:719–725. - PubMed
-
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 2006;96:305–313. - PubMed
-
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. - PubMed
-
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5'-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J.Biol.Chem. 2004;279:38441–38447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

