Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis
- PMID: 19196252
- PMCID: PMC2673743
- DOI: 10.1111/j.1365-2249.2009.03875.x
Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis
Abstract
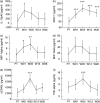
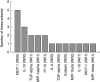
Biomarkers for treatment response would facilitate the testing of urgently needed new anti-tuberculous drugs. The present study investigated the profiles of 30 proinflammatory, anti-inflammatory and angiogenic factors [epidermal growth factor, eotaxin, fractalkine, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interleukin (IL)-1alpha, IL-1beta, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, interferon-gamma, interferon-inducible protein-10, Krebs von den Lungen-6, monocyte chemotactic protein-1, macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, sCD40L, transforming growth factor-alpha, tumour necrosis factor-alpha and vascular endothelial growth factor] in the plasma of 12 healthy tuberculin skin test-positive community controls and 20 human immunodeficiency virus-negative patients with active tuberculosis (TB) and identified potential biomarkers for early treatment response. We showed differences in the level of circulating cytokines between healthy controls and TB patients, but also between fast responders and slow responders to anti-tuberculosis treatment. The general discriminant analysis based on pre-treatment and week 1 measurements identified 10 sets of three-variable models that could classify fast and slow responders with up to 83% accuracy. Overall, this study shows the potential of cytokines as indicators of anti-tuberculosis treatment response.
Figures




References
-
- Cardona PJ, Ruiz-Manzano J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur Respir J. 2004;24:1044–51. - PubMed
-
- Global Tuberculosis Control: Surveillance. 2008. Planning, Financing: WHO report.
-
- World Health Organization. World Health Organization fact sheet. Geneva, Switzerland: World Health Organization; 2002.
-
- Enarson DE, Rieder HL, Arnadottir T, Trebucq A. Management of tuberculosis a guide for low income countries. 5th edn. Paris: IUATLD; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

