Into the blue: gene duplication and loss underlie color vision adaptations in a deep-sea chimaera, the elephant shark Callorhinchus milii
- PMID: 19196633
- PMCID: PMC2661800
- DOI: 10.1101/gr.084509.108
Into the blue: gene duplication and loss underlie color vision adaptations in a deep-sea chimaera, the elephant shark Callorhinchus milii
Abstract
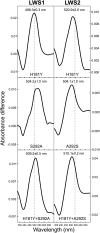
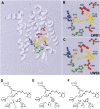
The cartilaginous fishes reside at the base of the gnathostome lineage as the oldest extant group of jawed vertebrates. Recently, the genome of the elephant shark, Callorhinchus milii, a chimaerid holocephalan, has been sequenced and therefore becomes the first cartilaginous fish to be analyzed in this way. The chimaeras have been largely neglected and very little is known about the visual systems of these fishes. By searching the elephant shark genome, we have identified gene fragments encoding a rod visual pigment, Rh1, and three cone visual pigments, the middle wavelength-sensitive or Rh2 pigment, and two isoforms of the long wavelength-sensitive or LWS pigment, LWS1 and LWS2, but no evidence for the two short wavelength-sensitive cone classes, SWS1 and SWS2. Expression of these genes in the retina was confirmed by RT-PCR. Full-length coding sequences were used for in vitro expression and gave the following peak absorbances: Rh1 496 nm, Rh2 442 nm, LWS1 499 nm, and LWS2 548 nm. Unusually, therefore, for a deep-sea fish, the elephant shark possesses cone pigments and the potential for trichromacy. Compared with other vertebrates, the elephant shark Rh2 and LWS1 pigments are the shortest wavelength-shifted pigments of their respective classes known to date. The mechanisms for this are discussed and we provide experimental evidence that the elephant shark LWS1 pigment uses a novel tuning mechanism to achieve the short wavelength shift to 499 nm, which inactivates the chloride-binding site. Our findings have important implications for the present knowledge of color vision evolution in early vertebrates.
Figures

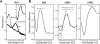

References
-
- Bellingham J., Tarttelin E.E., Foster R.G., Wells D.J. Structure and evolution of the teleost extraretinal rod-like opsin (errlo) and ocular rod opsin (rho) genes: Is teleost rho a retrogene? J. Exp. Zoolog. B Mol. Dev. Evol. 2003;297:1–10. - PubMed
-
- Bowmaker J.K., Govardovskii V.I., Shukolyukov S.A., Zueva L.V., Hunt D.M., Sideleva V.G., Smirnova O.G. Visual pigments and the photic environment: The cottoid fish of Lake Baikal. Vision Res. 1994;34:591–605. - PubMed
-
- Cappetta H., Duffin C., Zidek J. The fossil record 2. In: Benton M.J., editor. Chondrichthyes. Chapman and Hall; London, UK: 1993. pp. 593–609.
-
- Cohen J.L., Hueter R.E., Organisciak D.T. The presence of a porphyropsin-based visual pigment in the juvenile lemon shark (Negaprion brevirostris) Vision Res. 1990;30:1949–1953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
