Advancing age alters the contribution of calcium release from smooth endoplasmic reticulum stores in superior cervical ganglion cells
- PMID: 19196634
- PMCID: PMC2673896
- DOI: 10.1093/gerona/gln053
Advancing age alters the contribution of calcium release from smooth endoplasmic reticulum stores in superior cervical ganglion cells
Abstract
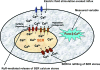
In superior cervical ganglion (SCG) neurons calcium-induced calcium release (CICR), mediated by ryanodine receptors (RyRs), contributes to stimulation-evoked intracellular calcium ([Ca(2+)](i)) transients.
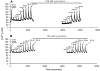
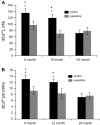
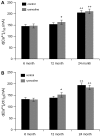
Hypothesis: The contribution of CICR to electrical field stimulation (EFS)-evoked [Ca(2+)](i) transients in SCG cells declines with senescence and may be partially recovered in the presence of caffeine. We measured EFS-evoked [Ca(2+)](i) transients in isolated fura-2-loaded SCG cells from Fischer-344 rats aged 6, 12, and 24 months with either the RyR antagonist ryanodine to block the contribution of CICR to [Ca(2+)](i) transients or caffeine to sensitize CICR to EFS. EFS-evoked [Ca(2+)](i) transients increased from 6 to 12 months and declined at 24 months and ryanodine decreased [Ca(2+)](i) transients in SCG cells from 6- and 12-month-old animals only. Caffeine significantly increased EFS-evoked [Ca(2+)](i) transients in all age groups. These data suggest that CICR declines with senescence and residual CICR function may be reclaimed in senescent cells with caffeine.
Figures




Similar articles
-
Maturation and long-term hypoxia alters Ca2+-induced Ca2+ release in sheep cerebrovascular sympathetic neurons.J Appl Physiol (1985). 2009 Oct;107(4):1223-34. doi: 10.1152/japplphysiol.00363.2009. Epub 2009 Jul 30. J Appl Physiol (1985). 2009. PMID: 19644029 Free PMC article.
-
Advancing age alters rapid and spontaneous refilling of caffeine-sensitive calcium stores in sympathetic superior cervical ganglion cells.J Appl Physiol (1985). 2005 Sep;99(3):963-71. doi: 10.1152/japplphysiol.00343.2005. Epub 2005 Apr 21. J Appl Physiol (1985). 2005. PMID: 15845773 Free PMC article.
-
Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones.J Physiol. 1997 Jul 1;502 ( Pt 1)(Pt 1):13-30. doi: 10.1111/j.1469-7793.1997.013bl.x. J Physiol. 1997. PMID: 9234194 Free PMC article.
-
Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle.Biochem Soc Trans. 2003 Oct;31(Pt 5):920-4. doi: 10.1042/bst0310920. Biochem Soc Trans. 2003. PMID: 14505449 Review.
-
Presence and functional significance of presynaptic ryanodine receptors.Prog Neurobiol. 2003 Apr;69(6):391-418. doi: 10.1016/s0301-0082(03)00053-4. Prog Neurobiol. 2003. PMID: 12880633 Review.
Cited by
-
Maturation and long-term hypoxia alters Ca2+-induced Ca2+ release in sheep cerebrovascular sympathetic neurons.J Appl Physiol (1985). 2009 Oct;107(4):1223-34. doi: 10.1152/japplphysiol.00363.2009. Epub 2009 Jul 30. J Appl Physiol (1985). 2009. PMID: 19644029 Free PMC article.
-
Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems.Am J Physiol Heart Circ Physiol. 2023 Nov 1;325(5):H1012-H1038. doi: 10.1152/ajpheart.00288.2023. Epub 2023 Aug 25. Am J Physiol Heart Circ Physiol. 2023. PMID: 37624095 Free PMC article. Review.
-
Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity.Pharmacol Ther. 2010 Feb;125(2):260-85. doi: 10.1016/j.pharmthera.2009.10.009. Epub 2009 Nov 25. Pharmacol Ther. 2010. PMID: 19931307 Free PMC article. Review.
References
-
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. - PubMed
-
- Faraci FM, Heistad DD. Regulation of cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. - PubMed
-
- Zhang B, Fugleholm K, Day LB, Ye S, Weller RO, Day IN. Molecular pathogenesis of subarachnoid hemorrhage. Int J Biochem Cell Biol. 2003;35:1341–1360. - PubMed
-
- Busija DW, Heistad DD, Marcus ML. Effects of sympathetic nerves on cerebral vessels during acute moderate increases in arterial pressures in dogs and cats. Circ Res. 1980;46:696–702. - PubMed
-
- Furuichi S, Endo S, Haji A, Takeda R, Nisijima M, Takaku A. Related changes in sympathetic activity, cerebral blood flow and intracranial pressure, and effect of an α-blocker in experimental subarachnoid haemorrhage. Acta Neurochir (Wien) 1999;141:415–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

