Maternal uterine vascular remodeling during pregnancy
- PMID: 19196652
- PMCID: PMC2760472
- DOI: 10.1152/physiol.00033.2008
Maternal uterine vascular remodeling during pregnancy
Abstract
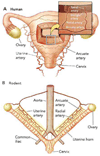
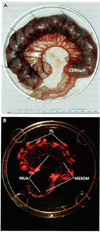
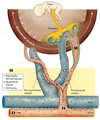
Sufficient uteroplacental blood flow is essential for normal pregnancy outcome and is accomplished by the coordinated growth and remodeling of the entire uterine circulation, as well as the creation of a new fetal vascular organ: the placenta. The process of remodeling involves a number of cellular processes, including hyperplasia and hypertrophy, rearrangement of existing elements, and changes in extracellular matrix. In this review, we provide information on uterine blood flow increases during pregnancy, the influence of placentation type on the distribution of uterine vascular resistance, consideration of the patterns, nature, and extent of maternal uterine vascular remodeling during pregnancy, and what is known about the underlying cellular mechanisms.
Figures


References
-
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. - PubMed
-
- Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol Cell Physiol. 1990;259:C484–C489. - PubMed
-
- Assali NS, Douglass RA, Jr, Baird WW, Nicholson DB, Suyemoto R. Measurement of uterine blood flow and uterine metabolism. IV. Results in normal pregnancy. Am J Obstet Gynecol. 1953;66:248–253. - PubMed
-
- Assali NS, Rauramo L, Peltonen T. Measurement of uterine blood flow and uterine metabolism VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am J Obstet Gynecol. 1960;79:86–98. - PubMed
-
- Ben Driss A, Benessiano J, Poitevin P, Levy BI, Michel JB. Arterial expansive remodeling induced by high flow rates. Am J Physiol Heart Circ Physiol. 1997;272:H851–H858. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

