Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid
- PMID: 19196800
- PMCID: PMC2654752
- DOI: 10.1210/en.2008-1437
Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid
Abstract
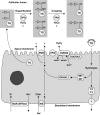
Thyroid hormones are essential for normal development and metabolism. Thyroid hormone biosynthesis requires iodide uptake into the thyrocytes and efflux into the follicular lumen, where it is organified on selected tyrosyls of thyroglobulin. Uptake of iodide into the thyrocytes is mediated by an intrinsic membrane glycoprotein, the sodium-iodide symporter (NIS), which actively cotransports two sodium cations per each iodide anion. NIS-mediated transport of iodide is driven by the electrochemical sodium gradient generated by the Na(+)/K(+)-ATPase. NIS is expressed in the thyroid, the salivary glands, gastric mucosa, and the lactating mammary gland. TSH and iodide regulate iodide accumulation by modulating NIS activity via transcriptional and posttranscriptional mechanisms. Biallelic mutations in the NIS gene lead to a congenital iodide transport defect, an autosomal recessive condition characterized by hypothyroidism, goiter, low thyroid iodide uptake, and a low saliva/plasma iodide ratio. Pendrin is an anion transporter that is predominantly expressed in the inner ear, the thyroid, and the kidney. Biallelic mutations in the SLC26A4 gene lead to Pendred syndrome, an autosomal recessive disorder characterized by sensorineural deafness, goiter, and impaired iodide organification. In thyroid follicular cells, pendrin is expressed at the apical membrane. Functional in vitro data and the impaired iodide organification observed in patients with Pendred syndrome support a role of pendrin as an apical iodide transporter.
Figures
References
-
- Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N 2003 The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 - PubMed
-
- Kopp P 2005 Thyroid hormone synthesis: thyroid iodine metabolism. In: Braverman L, Utiger R, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 9th ed. New York: Lippincott Williams Wilkins; 52–76
-
- Arvan P, Di Jeso B 2005 Thyroglobulin structure, function, and biosynthesis. In: Braverman L, Utiger R, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. New York: Lippincott Williams Wilkins; 77–95
-
- Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED 2000 Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845 - PubMed
-
- Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP 1999 The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21:440–443 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

