Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice
- PMID: 19196849
- PMCID: PMC2683394
- DOI: 10.1124/dmd.108.023317
Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice
Abstract
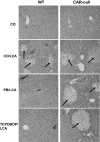
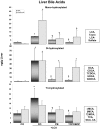
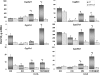
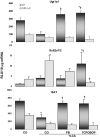
Pharmacological activation of the constitutive androstane receptor (CAR) protects the liver during cholestasis. The current study evaluates how activation of CAR influences genes involved in bile acid biosynthesis as a mechanism of hepatoprotection during bile acid-induced liver injury. CAR activators phenobarbital (PB) and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) or corn oil (CO) were administered to C57BL/6 wild-type (WT) and CAR knockout (CAR-null) mice before and during induction of intrahepatic cholestasis using the secondary bile acid, lithocholic acid (LCA). In LCA-treated WT and all the CAR-null groups (excluding controls), histology revealed severe multifocal necrosis. This pathology was absent in WT mice pretreated with PB and TCPOBOP, indicating CAR-dependent hepatoprotection. Decreases in total hepatic bile acids and hepatic monohydroxy, dihydroxy, and trihydroxy bile acids in PB- and TCPOBOP-pretreated WT mice correlated with hepatoprotection. In comparison, concentrations of monohydroxylated and dihydroxylated bile acids were increased in all the treated CAR-null mice compared with CO controls. Along with several other enzymes (Cyp7b1, Cyp27a1, Cyp39a1), Cyp8b1 expression was increased in hepatoprotected mice, which could be suggestive of a shift in the bile acid biosynthesis pathway toward the formation of less toxic bile acids. In CAR-null mice, these changes in gene expression were not different among treatment groups. These results suggest CAR mediates a shift in bile acid biosynthesis toward the formation of less toxic bile acids, as well as a decrease in hepatic bile acid concentrations. We propose that these combined CAR-mediated effects may contribute to the hepatoprotection observed during LCA-induced liver injury.
Figures


References
-
- Alnouti Y and Klaassen CD (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93 242–255. - PubMed
-
- Annapurna V, Mukundan M, Sesikeran B, and Bamji M (1989) Effects of ovulen-50, diethylnitrosamine and phenobarbital on liver regeneration in female rats. J Biosci 14 1–7.
-
- Araya Z and Wikvall K (1999) 6alpha-Hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta 1438 47–54. - PubMed
-
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and SULT2a1/2 as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279 22250–22257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

