One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial
- PMID: 19196887
- PMCID: PMC2671094
- DOI: 10.2337/dc08-1797
One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial
Abstract
Objective: Traditional blood glucose-lowering agents do not sustain adequate glycemic control in most type 2 diabetic patients. Preclinical studies with exenatide have suggested sustained improvements in beta-cell function. We investigated the effects of 52 weeks of treatment with exenatide or insulin glargine followed by an off-drug period on hyperglycemic clamp-derived measures of beta-cell function, glycemic control, and body weight.
Research design and methods: Sixty-nine metformin-treated patients with type 2 diabetes were randomly assigned to exenatide (n = 36) or insulin glargine (n = 33). beta-Cell function was measured during an arginine-stimulated hyperglycemic clamp at week 0, at week 52, and after a 4-week off-drug period. Additional end points included effects on glycemic control, body weight, and safety.
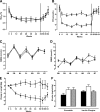
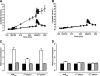
Results: Treatment-induced change in combined glucose- and arginine-stimulated C-peptide secretion was 2.46-fold (95% CI 2.09-2.90, P < 0.0001) greater after a 52-week exenatide treatment compared with insulin glargine treatment. Both exenatide and insulin glargine reduced A1C similarly: -0.8 +/- 0.1 and -0.7 +/- 0.2%, respectively (P = 0.55). Exenatide reduced body weight compared with insulin glargine (difference -4.6 kg, P < 0.0001). beta-Cell function measures returned to pretreatment values in both groups after a 4-week off-drug period. A1C and body weight rose to pretreatment values 12 weeks after discontinuation of either exenatide or insulin glargine therapy.
Conclusions: Exenatide significantly improves beta-cell function during 1 year of treatment compared with titrated insulin glargine. After cessation of both exenatide and insulin glargine therapy, beta-cell function and glycemic control returned to pretreatment values, suggesting that ongoing treatment is necessary to maintain the beneficial effects of either therapy.
Figures

References
-
- Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3– 19 - PubMed
-
- Riddle MC: Timely initiation of basal insulin. Am J Med 2004; 116: 3S– 9S - PubMed
-
- Turner RC, Cull CA, Frighi V, Holman RR: the UKPDSG. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281: 2005– 2012 - PubMed
-
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD: the Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628– 2635 - PubMed
-
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092– 1100 - PubMed

