Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling
- PMID: 19196953
- PMCID: PMC2650341
- DOI: 10.1073/pnas.0807694106
Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling
Abstract
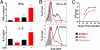
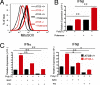
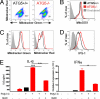
Autophagy is a highly conserved process that maintains homeostasis by clearing damaged organelles and long-lived proteins. The consequences of deficiency in autophagy manifest in a variety of pathological states including neurodegenerative diseases, inflammatory disorders, and cancer. Here, we studied the role of autophagy in the homeostatic regulation of innate antiviral defense. Single-stranded RNA viruses are recognized by the members of the RIG-I-like receptors (RLRs) in the cytosol. RLRs signal through IPS-1, resulting in the production of the key antiviral cytokines, type I IFNs. Autophagy-defective Atg5(-/-) cells exhibited enhanced RLR signaling, increased IFN secretion, and resistance to infection by vesicular stomatitis virus. In the absence of autophagy, cells accumulated dysfunctional mitochondria, as well as mitochondria-associated IPS-1. Reactive oxygen species (ROS) associated with the dysfunctional mitochondria were largely responsible for the enhanced RLR signaling in Atg5(-/-) cells, as antioxidant treatment blocked the excess RLR signaling. In addition, autophagy-independent increase in mitochondrial ROS by treatment of cells with rotenone was sufficient to amplify RLR signaling in WT cells. These data indicate that autophagy contributes to homeostatic regulation of innate antiviral defense through the clearance of dysfunctional mitochondria, and revealed that ROS associated with mitochondria play a key role in potentiating RLR signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

