Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B
- PMID: 19196955
- PMCID: PMC2650359
- DOI: 10.1073/pnas.0810530106
Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B
Abstract
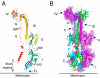
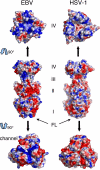
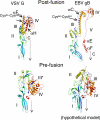
Epstein-Barr virus (EBV) is a herpesvirus that is associated with development of malignancies of lymphoid tissue. EBV infections are life-long and occur in >90% of the population. Herpesviruses enter host cells in a process that involves fusion of viral and cellular membranes. The fusion apparatus is comprised of envelope glycoprotein B (gB) and a heterodimeric complex made of glycoproteins H and L. Glycoprotein B is the most conserved envelope glycoprotein in human herpesviruses, and the structure of gB from Herpes simplex virus 1 (HSV-1) is available. Here, we report the crystal structure of the secreted EBV gB ectodomain, which forms 16-nm long spike-like trimers, structurally homologous to the postfusion trimers of the fusion protein G of vesicular stomatitis virus (VSV). Comparative structural analyses of EBV gB and VSV G, which has been solved in its pre and postfusion states, shed light on gB residues that may be involved in conformational changes and membrane fusion. Also, the EBV gB structure reveals that, despite the high sequence conservation of gB in herpesviruses, the relative orientations of individual domains, the surface charge distributions, and the structural details of EBV gB differ from the HSV-1 protein, indicating regions and residues that may have important roles in virus-specific entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pellet PE, Roizman B. In: Fields Virology. Knipe DM, Howley PM, editors. New York: Lippincott Williams and Wilkins; 2007. pp. 2479–2499.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

