Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin
- PMID: 19196969
- PMCID: PMC2650375
- DOI: 10.1073/pnas.0900008106
Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin
Abstract
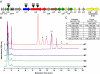
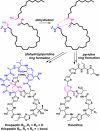
The thiazolylpeptides are a family of >50 bactericidal antibiotics that block the initial steps of bacterial protein synthesis. Here, we report a biosynthetic gene cluster for thiocillin and establish that it, and by extension the whole class, is ribosomally synthesized. Remarkably, the C-terminal 14 residues of a 52-residue peptide precursor undergo 13 posttranslational modifications to give rise to thiocillin, making this antibiotic the most heavily posttranslationally-modified peptide known to date.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Baldwin JE, Abraham E. The biosynthesis of penicillins and cephalosporins. Natural Product Rep. 1988;5:129–145. - PubMed
-
- Donadio S, Sosio M, Stegmann E, Weber T, Wohlleben W. Comparative analysis and insights into the evolution of gene clusters for glycopeptide antibiotic biosynthesis. Mol Genet Genomics. 2005;274:40–50. - PubMed
-
- Baltz RH. Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr Top Med Chem. 2008;8:618–638. - PubMed
-
- Finking R, Marahiel MA. Biosynthesis of nonribosomal peptides 1. Annu Rev Microbiol. 2004;58:453–488. - PubMed
-
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: Logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

