The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma
- PMID: 19196975
- PMCID: PMC2636735
- DOI: 10.1073/pnas.0809579106
The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma
Abstract
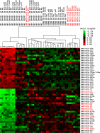
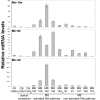
Medulloblastomas (MBs) are the most common brain tumors in children. Some are thought to originate from cerebellar granule neuron progenitors (GNPs) that fail to undergo normal cell cycle exit and differentiation. Because microRNAs regulate numerous aspects of cellular physiology and development, we reasoned that alterations in miRNA expression might contribute to MB. We tested this hypothesis using 2 spontaneous mouse MB models with specific initiating mutations, Ink4c-/-; Ptch1+/- and Ink4c-/-; p53-/-. We found that 26 miRNAs showed increased expression and 24 miRNAs showed decreased expression in proliferating mouse GNPs and MBs relative to mature mouse cerebellum, regardless of genotype. Among the 26 overexpressed miRNAs, 9 were encoded by the miR-17 approximately 92 cluster family, a group of microRNAs implicated as oncogenes in several tumor types. Analysis of human MBs demonstrated that 3 miR-17 approximately 92 cluster miRNAs (miR-92, miR-19a, and miR-20) were also overexpressed in human MBs with a constitutively activated Sonic Hedgehog (SHH) signaling pathway, but not in other forms of the disease. To test whether the miR-17 approximately 92 cluster could promote MB formation, we enforced expression of these miRNAs in GNPs isolated from cerebella of postnatal (P) day P6 Ink4c-/-; Ptch1+/- mice. These, but not similarly engineered cells from Ink4c-/-; p53-/- mice, formed MBs in orthotopic transplants with complete penetrance. Interestingly, orthotopic mouse tumors ectopically expressing miR-17 approximately 92 lost expression of the wild-type Ptch1 allele. Our findings suggest a functional collaboration between the miR-17 approximately 92 cluster and the SHH signaling pathway in the development of MBs in mouse and man.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation.Genes Dev. 2005 Nov 15;19(22):2656-67. doi: 10.1101/gad.1368605. Epub 2005 Oct 31. Genes Dev. 2005. PMID: 16260494 Free PMC article.
-
Ex vivo miRNome analysis in Ptch1+/- cerebellum granule cells reveals a subset of miRNAs involved in radiation-induced medulloblastoma.Oncotarget. 2016 Oct 18;7(42):68253-68269. doi: 10.18632/oncotarget.11938. Oncotarget. 2016. PMID: 27626168 Free PMC article.
-
The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors.Cancer Res. 2009 Apr 15;69(8):3249-55. doi: 10.1158/0008-5472.CAN-08-4710. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351822 Free PMC article.
-
The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma.Mol Brain. 2015 Oct 17;8(1):64. doi: 10.1186/s13041-015-0155-5. Mol Brain. 2015. PMID: 26475605 Free PMC article. Review.
-
Sonic hedgehog patterning during cerebellar development.Cell Mol Life Sci. 2016 Jan;73(2):291-303. doi: 10.1007/s00018-015-2065-1. Epub 2015 Oct 24. Cell Mol Life Sci. 2016. PMID: 26499980 Free PMC article. Review.
Cited by
-
MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke.J Biol Chem. 2013 May 3;288(18):12478-88. doi: 10.1074/jbc.M112.449025. Epub 2013 Mar 19. J Biol Chem. 2013. PMID: 23511639 Free PMC article.
-
Utility of Lymphoblastoid Cell Lines for Induced Pluripotent Stem Cell Generation.Stem Cells Int. 2016;2016:2349261. doi: 10.1155/2016/2349261. Epub 2016 Jun 7. Stem Cells Int. 2016. PMID: 27375745 Free PMC article.
-
Medulloblastoma epigenetics and the path to clinical innovation.J Neurooncol. 2020 Oct;150(1):35-46. doi: 10.1007/s11060-020-03591-9. Epub 2020 Aug 20. J Neurooncol. 2020. PMID: 32816225 Free PMC article. Review.
-
The clinical implications of medulloblastoma subgroups.Nat Rev Neurol. 2012 May 8;8(6):340-51. doi: 10.1038/nrneurol.2012.78. Nat Rev Neurol. 2012. PMID: 22565209 Review.
-
miR-17-92 expression in differentiated T cells - implications for cancer immunotherapy.J Transl Med. 2010 Feb 18;8:17. doi: 10.1186/1479-5876-8-17. J Transl Med. 2010. PMID: 20167088 Free PMC article.
References
-
- Oliver TG, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medullolastoma. Development. 2005;132:2425–2439. - PubMed
-
- Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. - PubMed
-
- Herzberg JJ, Wiskemann A. Basal cell nevus with hereditary malformation and medulloblastoma. Dermatologica. 1963;126:106–123. - PubMed
-
- Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–5928. - PubMed
-
- Hahn H, et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental anomalies. J Biol Chem. 1996;271:12125–12128. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

