Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood
- PMID: 19197072
- PMCID: PMC2669404
- DOI: 10.1189/jlb.0208145
Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood
Abstract
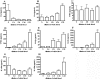
Several observations suggest endogenous suppressors of inflammatory mediators are present in human blood. alpha-1-Antitrypsin (AAT) is the most abundant serine protease inhibitor in blood, and AAT possesses anti-inflammatory activity in vitro and in vivo. Here, we show that in vitro stimulation of whole blood from persons with a genetic AAT deficiency resulted in enhanced cytokine production compared with blood from healthy subjects. Using whole blood from healthy subjects, dilution of blood with RPMI tissue-culture medium, followed by incubation for 18 h, increased spontaneous production of IL-8, TNF-alpha, IL-1 beta, and IL-1R antagonist (IL-1Ra) significantly, compared with undiluted blood. Dilution-induced cytokine production suggested the presence of one or more circulating inhibitors of cytokine synthesis present in blood. Serially diluting blood with tissue-culture medium in the presence of cytokine stimulation with heat-killed Staphylococcus epidermidis (S. epi) resulted in 1.2- to 55-fold increases in cytokine production compared with S. epi stimulation alone. Diluting blood with autologous plasma did not increase the production of IL-8, TNF-alpha, IL-1 beta, or IL-1Ra, suggesting that the endogenous, inhibitory activity of blood resided in plasma. In whole blood, diluted and stimulated with S. epi, exogenous AAT inhibited IL-8, IL-6, TNF-alpha, and IL-1 beta significantly but did not suppress induction of the anti-inflammatory cytokines IL-1Ra and IL-10. These ex vivo and in vitro observations suggest that endogenous AAT in blood contributes to the suppression of proinflammatory cytokine synthesis.
Figures
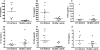
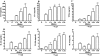

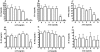

Similar articles
-
Levels of pro-inflammatory cytokines produced from cord blood in-vitro are pathogen dependent and increased in comparison to adult controls.Cytokine. 2007 Sep;39(3):171-7. doi: 10.1016/j.cyto.2007.07.004. Epub 2007 Sep 19. Cytokine. 2007. PMID: 17884557
-
Steroids induce a disequilibrium of secreted interleukin-1 receptor antagonist and interleukin-1β synthesis by human neutrophils.Eur Respir J. 2011 Feb;37(2):406-15. doi: 10.1183/09031936.00170409. Epub 2010 Jul 22. Eur Respir J. 2011. PMID: 20650986
-
Whole blood production of monocytic cytokines (IL-1beta, IL-6, TNF-alpha, sIL-6R, IL-1Ra) in haemodialysed patients.Nephrol Dial Transplant. 1999 Oct;14(10):2420-6. doi: 10.1093/ndt/14.10.2420. Nephrol Dial Transplant. 1999. PMID: 10528667
-
Lipopolysaccharide from Actinobacillus actinomycetemcomitans stimulates production of interleukin-1beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-1 receptor antagonist in human whole blood.J Periodontal Res. 1999 Jan;34(1):34-40. doi: 10.1111/j.1600-0765.1999.tb02219.x. J Periodontal Res. 1999. PMID: 10086884
-
Age-dependent changes of proinflammatory cytokine production by porcine peripheral blood phagocytes.Vet Immunol Immunopathol. 2008 Aug 15;124(3-4):367-78. doi: 10.1016/j.vetimm.2008.04.016. Epub 2008 Apr 30. Vet Immunol Immunopathol. 2008. PMID: 18534689
Cited by
-
Does Genetic Predisposition Contribute to the Exacerbation of COVID-19 Symptoms in Individuals with Comorbidities and Explain the Huge Mortality Disparity between the East and the West?Int J Mol Sci. 2021 May 8;22(9):5000. doi: 10.3390/ijms22095000. Int J Mol Sci. 2021. PMID: 34066804 Free PMC article. Review.
-
A randomized, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for ARDS secondary to COVID-19.Med. 2022 Apr 8;3(4):233-248.e6. doi: 10.1016/j.medj.2022.03.001. Epub 2022 Mar 11. Med. 2022. PMID: 35291694 Free PMC article. Clinical Trial.
-
Engineering the serpin α1 -antitrypsin: A diversity of goals and techniques.Protein Sci. 2020 Apr;29(4):856-871. doi: 10.1002/pro.3794. Epub 2019 Dec 9. Protein Sci. 2020. PMID: 31774589 Free PMC article. Review.
-
Assessing inflammatory protein biomarkers in COPD subjects with and without alpha-1 antitrypsin deficiency.Respir Res. 2025 Jul 15;26(1):247. doi: 10.1186/s12931-025-03320-8. Respir Res. 2025. PMID: 40665347 Free PMC article.
-
Toll-like receptor signaling pathway triggered by inhibition of serpin A1 stimulates production of inflammatory cytokines by endometrial stromal cells.Front Endocrinol (Lausanne). 2022 Aug 24;13:966455. doi: 10.3389/fendo.2022.966455. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093086 Free PMC article.
References
-
- Chernoff A E, Granowitz E V, Shapiro L, Vannier E, Lonnemann G, Angel J B, Kennedy J S, Rabson A R, Wolff S M, Dinarello C A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995;154:5492–5499. - PubMed
-
- Godoy-Ramirez K, Franck K, Mahdavifar S, Andersson L, Gaines H. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J Immunol Methods. 2004;292:1–15. - PubMed
-
- Sewell W A, North M E, Webster A D, Farrant J. Determination of intracellular cytokines by flow-cytometry following whole-blood culture. J Immunol Methods. 1997;209:67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

