Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents
- PMID: 19197140
- PMCID: PMC2648677
- DOI: 10.1172/JCI35070
Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents
Abstract
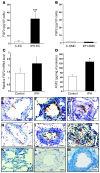
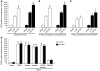
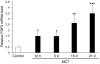
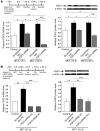
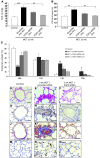
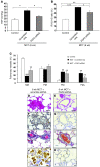
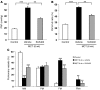
Pulmonary hypertension (PH) is a progressive, lethal lung disease characterized by pulmonary artery SMC (PA-SMC) hyperplasia leading to right-sided heart failure. Molecular events originating in pulmonary ECs (P-ECs) may contribute to the PA-SMC hyperplasia in PH. Thus, we exposed cultured human PA-SMC to medium conditioned by P-EC from patients with idiopathic PH (IPH) or controls and found that IPH P-EC-conditioned medium increased PA-SMC proliferation more than control P-EC medium. Levels of FGF2 were increased in the medium of IPH P-ECs over controls, while there was no detectable difference in TGF-beta1, PDGF-BB, or EGF levels. No difference in FGF2-induced proliferation or FGF receptor type 1 (FGFR1) mRNA levels was detected between IPH and control PA-SMCs. Knockdown of FGF2 in P-EC using siRNA reduced the PA-SMC growth-stimulating effects of IPH P-EC medium by 60% and control P-EC medium by 10%. In situ hybridization showed FGF2 overproduction predominantly in the remodeled vascular endothelium of lungs from patients with IPH. Repeated intravenous FGF2-siRNA administration abolished lung FGF2 production, both preventing and nearly reversing a rat model of PH. Similarly, pharmacological FGFR1 inhibition with SU5402 reversed established PH in the same model. Thus, endothelial FGF2 is overproduced in IPH and contributes to SMC hyperplasia in IPH, identifying FGF2 as a promising target for new treatments against PH.
Figures

References
-
- Humbert M., et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43:13S–24S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

