Prevention of KLF4-mediated tumor initiation and malignant transformation by UAB30 rexinoid
- PMID: 19197145
- PMCID: PMC2776760
- DOI: 10.4161/cbt.8.3.7486
Prevention of KLF4-mediated tumor initiation and malignant transformation by UAB30 rexinoid
Abstract
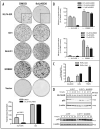
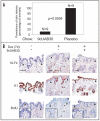
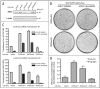
The transcription factor KLF4 acts in post-mitotic epithelial cells to promote differentiation and functions in a context-dependent fashion as an oncogene. In the skin KLF4 is co-expressed with the nuclear receptors RARgamma and RXRalpha, and formation of the skin permeability barrier is a shared function of these three proteins. We utilized a KLF4-transgenic mouse model of skin cancer in combination with cultured epithelial cells to examine functional interactions between KLF4 and retinoic acid receptors. In cultured cells, activation of a conditional, KLF4-estrogen receptor fusion protein by 4-hydroxytamoxifen resulted in rapid upregulation of transcripts for nuclear receptors including RARgamma and RXRalpha. We tested retinoids in epithelial cell transformation assays, including an RAR-selective agonist (all-trans RA), an RXR-selective agonist (9-cis UAB30, rexinoid), and a pan agonist (9-cis RA). Unlike for several other genes, transformation by KLF4 was inhibited by each retinoid, implicating distinct nuclear receptor heterodimers as modulators of KLF4 transforming activity. When RXRalpha expression was suppressed by RNAi in cultured cells, transformation was promoted and the inhibitory effect of 9-cis UAB30 was attenuated. Similarly as shown for other mouse models of skin cancer, rexinoid prevented skin tumor initiation resulting from induction of KLF4 in basal keratinocytes. Rexinoid permitted KLF4 expression and KLF4-induced cell cycling, but attenuated the KLF4-induced misexpression of cytokeratin 1 in basal cells. Neoplastic lesions including hyperplasia, dysplasia and squamous cell carcinoma-like lesions were prevented for up to 30 days. Taken together, the results identify retinoid receptors including RXRalpha as ligand-dependent inhibitors of KLF4-mediated transformation or tumorigenesis.
Figures


Similar articles
-
KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia.Cancer Biol Ther. 2005 Dec;4(12):1401-8. doi: 10.4161/cbt.4.12.2355. Epub 2005 Dec 23. Cancer Biol Ther. 2005. PMID: 16357510 Free PMC article.
-
Synergistic activation of retinoic acid (RA)-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha (RAR alpha)-, RAR beta-, or RAR gamma-selective ligand in combination with a retinoid X receptor-specific ligand.Mol Cell Biol. 1995 Dec;15(12):6481-7. doi: 10.1128/MCB.15.12.6481. Mol Cell Biol. 1995. PMID: 8524212 Free PMC article.
-
Endogenous retinoic acid receptor (RAR)-retinoid X receptor (RXR) heterodimers are the major functional forms regulating retinoid-responsive elements in adult human keratinocytes. Binding of ligands to RAR only is sufficient for RAR-RXR heterodimers to confer ligand-dependent activation of hRAR beta 2/RARE (DR5).J Biol Chem. 1995 Feb 17;270(7):3001-11. doi: 10.1074/jbc.270.7.3001. J Biol Chem. 1995. PMID: 7852380
-
Cyclin proteolysis as a retinoid cancer prevention mechanism.Ann N Y Acad Sci. 2001 Dec;952:13-22. doi: 10.1111/j.1749-6632.2001.tb02724.x. Ann N Y Acad Sci. 2001. PMID: 11795432 Review.
-
F9 embryocarcinoma cells: a cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling.Histol Histopathol. 2001 Jul;16(3):909-22. doi: 10.14670/HH-16.909. Histol Histopathol. 2001. PMID: 11510982 Review.
Cited by
-
Retinoid X Receptor Agonists Upregulate Genes Responsible for the Biosynthesis of All-Trans-Retinoic Acid in Human Epidermis.PLoS One. 2016 Apr 14;11(4):e0153556. doi: 10.1371/journal.pone.0153556. eCollection 2016. PLoS One. 2016. PMID: 27078158 Free PMC article.
-
Defining the communication between agonist and coactivator binding in the retinoid X receptor α ligand binding domain.J Biol Chem. 2014 Jan 10;289(2):814-26. doi: 10.1074/jbc.M113.476861. Epub 2013 Nov 1. J Biol Chem. 2014. PMID: 24187139 Free PMC article.
-
A KLF4-miRNA-206 autoregulatory feedback loop can promote or inhibit protein translation depending upon cell context.Mol Cell Biol. 2011 Jun;31(12):2513-27. doi: 10.1128/MCB.01189-10. Epub 2011 Apr 25. Mol Cell Biol. 2011. PMID: 21518959 Free PMC article.
-
Nuclear hormone receptor functions in keratinocyte and melanocyte homeostasis, epidermal carcinogenesis and melanomagenesis.FEBS Lett. 2013 Mar 18;587(6):529-41. doi: 10.1016/j.febslet.2013.01.041. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395795 Free PMC article. Review.
-
A Randomized, Placebo-Controlled, Double-Blind, Dose Escalation, Single Dose, and Steady-State Pharmacokinetic Study of 9cUAB30 in Healthy Volunteers.Cancer Prev Res (Phila). 2019 Dec;12(12):903-912. doi: 10.1158/1940-6207.CAPR-19-0310. Epub 2019 Sep 4. Cancer Prev Res (Phila). 2019. PMID: 31484659 Free PMC article. Clinical Trial.
References
-
- Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK, Frost AR, Louro ID, Townes TM, Paterson AJ, Kudlow JE, Lobo-Ruppert SM, Ruppert JM. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24:1491–500. - PMC - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
