TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells
- PMID: 19197243
- PMCID: PMC2666037
- DOI: 10.1038/emboj.2009.8
TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells
Erratum in
- EMBO J. 2009 May 20;28(10):1532
Abstract
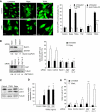
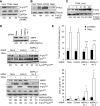
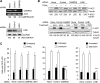
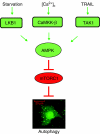
The capacity of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) to trigger apoptosis preferentially in cancer cells, although sparing normal cells, has motivated clinical development of TRAIL receptor agonists as anti-cancer therapeutics. The molecular mechanisms responsible for the differential TRAIL sensitivity of normal and cancer cells are, however, poorly understood. Here, we show a novel signalling pathway that activates cytoprotective autophagy in untransformed human epithelial cells treated with TRAIL. TRAIL-induced autophagy is mediated by the AMP-activated protein kinase (AMPK) that inhibits mammalian target of rapamycin complex 1, a potent inhibitor of autophagy. Interestingly, the TRAIL-induced AMPK activation is refractory to the depletion of the two known AMPK-activating kinases, LKB1 and Ca(2+)/calmodulin-dependent kinase kinase-beta, but depends on transforming growth factor-beta-activating kinase 1 (TAK1) and TAK1-binding subunit 2. As TAK1 and AMPK are ubiquitously expressed kinases activated by numerous cytokines and developmental cues, these data are most likely to have broad implications for our understanding of cellular control of energy homoeostasis as well as the resistance of untransformed cells against TRAIL-induced apoptosis.
Figures

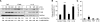

References
-
- Adhikari A, Xu M, Chen ZJ (2007) Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26: 3214–3226 - PubMed
-
- Brenman JE (2007) AMPK/LKB1 signaling in epithelial cell polarity and cell division. Cell Cycle 6: 2755–2759 - PubMed
-
- Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12 (Suppl 2): 1509–1518 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

