The 3Ms of central spindle assembly: microtubules, motors and MAPs
- PMID: 19197328
- PMCID: PMC2789570
- DOI: 10.1038/nrm2609
The 3Ms of central spindle assembly: microtubules, motors and MAPs
Abstract
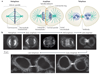
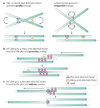
During metaphase, sister chromatids are positioned at the midpoint of the microtubule-based mitotic spindle in preparation for their segregation. The onset of anaphase triggers inactivation of the key mitotic kinase cyclin-dependent kinase 1 (CDK1) and the polewards movement of sister chromatids. During anaphase, the mitotic spindle reorganizes in preparation for cytokinesis. Kinesin motor proteins and microtubule-associated proteins bundle the plus ends of interpolar microtubules and generate the central spindle, which regulates cleavage furrow initiation and the completion of cytokinesis. Complementary approaches, including cell biology, genetics and computational modelling, have provided new insights into the mechanism and regulation of central spindle assembly.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

