Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation
- PMID: 19197350
- PMCID: PMC2629125
- DOI: 10.1371/journal.ppat.1000287
Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation
Abstract
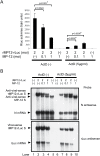
Rift Valley fever virus (RVFV) (genus Phlebovirus, family Bunyaviridae) is a negative-stranded RNA virus with a tripartite genome. RVFV is transmitted by mosquitoes and causes fever and severe hemorrhagic illness among humans, and fever and high rates of abortions in livestock. A nonstructural RVFV NSs protein inhibits the transcription of host mRNAs, including interferon-beta mRNA, and is a major virulence factor. The present study explored a novel function of the RVFV NSs protein by testing the replication of RVFV lacking the NSs gene in the presence of actinomycin D (ActD) or alpha-amanitin, both of which served as a surrogate of the host mRNA synthesis suppression function of the NSs. In the presence of the host-transcriptional inhibitors, the replication of RVFV lacking the NSs protein, but not that carrying NSs, induced double-stranded RNA-dependent protein kinase (PKR)-mediated eukaryotic initiation factor (eIF)2alpha phosphorylation, leading to the suppression of host and viral protein translation. RVFV NSs promoted post-transcriptional downregulation of PKR early in the course of the infection and suppressed the phosphorylated eIF2alpha accumulation. These data suggested that a combination of RVFV replication and NSs-induced host transcriptional suppression induces PKR-mediated eIF2alpha phosphorylation, while the NSs facilitates efficient viral translation by downregulating PKR and inhibiting PKR-mediated eIF2alpha phosphorylation. Thus, the two distinct functions of the NSs, i.e., the suppression of host transcription, including that of type I interferon mRNAs, and the downregulation of PKR, work together to prevent host innate antiviral functions, allowing efficient replication and survival of RVFV in infected mammalian hosts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
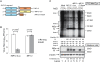




References
-
- Peters CJ. Rift Valley fever. Handbook Series of Zoonoses, Section B: Viral Zoonoses, Vol 1. CRC Press; 1981.
-
- Morvan J, Rollin PE, Laventure S, Rakotoarivony I, Roux J. Rift Valley fever epizootic in the central highlands of Madagascar. Res Virol. 1992;143:407–415. - PubMed
-
- Meegan JM. The Rift Valley fever epizootic in Egypt 1977–78. 1. Description of the epizootic and virological studies. Trans R Soc Trop Med Hyg. 1979;73:618–623. - PubMed
-
- El-Akkad AM. Rift Valley fever outbreak in Egypt. October–December 1977. The Journal of the Egyptian Public Health Association. 1978;53:123–128. - PubMed
-
- Arthur RR, el-Sharkawy MS, Cope SE, Botros BA, Oun S, et al. Recurrence of Rift Valley fever in Egypt. Lancet. 1993;342:1149–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

