Deletion of the mitochondrial flavoprotein apoptosis inducing factor (AIF) induces beta-cell apoptosis and impairs beta-cell mass
- PMID: 19197367
- PMCID: PMC2632884
- DOI: 10.1371/journal.pone.0004394
Deletion of the mitochondrial flavoprotein apoptosis inducing factor (AIF) induces beta-cell apoptosis and impairs beta-cell mass
Erratum in
-
Correction: Deletion of the Mitochondrial Flavoprotein Apoptosis Inducing Factor (AIF) Induces β-Cell Apoptosis and Impairs β-Cell Mass.PLoS One. 2015 May 8;10(5):e0117766. doi: 10.1371/journal.pone.0117766. eCollection 2015. PLoS One. 2015. PMID: 25954805 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Deletion of the Mitochondrial Flavoprotein Apoptosis Inducing Factor (AIF) Induces β-Cell Apoptosis and Impairs β-Cell Mass.PLoS One. 2022 Aug 25;17(8):e0272901. doi: 10.1371/journal.pone.0272901. eCollection 2022. PLoS One. 2022. PMID: 36006897 Free PMC article. No abstract available.
Abstract
Background: Apoptosis is a hallmark of beta-cell death in both type 1 and type 2 diabetes mellitus. Understanding how apoptosis contributes to beta-cell turnover may lead to strategies to prevent progression of diabetes. A key mediator of apoptosis, mitochondrial function, and cell survival is apoptosis inducing factor (AIF). In the present study, we investigated the role of AIF on beta-cell mass and survival using the Harlequin (Hq) mutant mice, which are hypomorphic for AIF.
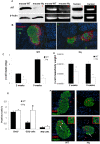
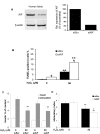
Methodology/principal findings: Immunohistochemical evaluation of pancreata from Hq mutant mice displayed much smaller islets compared to wild-type mice (WT). Analysis of beta-cell mass in these mice revealed a greater than 4-fold reduction in beta-cell mass together with an 8-fold increase in beta-cell apoptosis. Analysis of cell cycle dynamics, using BrdU pulse as a marker for cells in S-phase, did not detect significant differences in the frequency of beta-cells in S-phase. In contrast, double staining for phosphorylated Histone H3 and insulin showed a 3-fold increase in beta-cells in the G2 phase in Hq mutant mice, but no differences in M-phase compared to WT mice. This suggests that the beta-cells from Hq mutant mice are arrested in the G2 phase and are unlikely to complete the cell cycle. beta-cells from Hq mutant mice display increased sensitivity to hydrogen peroxide-induced apoptosis, which was confirmed in human islets in which AIF was depleted by siRNA. AIF deficiency had no effect on glucose stimulated insulin secretion, but the impaired effect of hydrogen peroxide on beta-cell function was potentiated.
Conclusions/significance: Our results indicate that AIF is essential for maintaining beta-cell mass and for oxidative stress response. A decrease in the oxidative phosphorylation capacity may counteract the development of diabetes, despite its deleterious effects on beta-cell survival.
Conflict of interest statement
Figures

References
-
- Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. - PubMed
-
- Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–2649. - PubMed
-
- Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium 2008 - PubMed
-
- Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, et al. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

