Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids
- PMID: 19197369
- PMCID: PMC2632888
- DOI: 10.1371/journal.ppat.1000289
Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids
Abstract
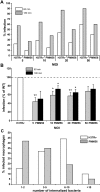
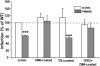
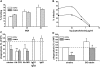
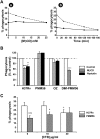
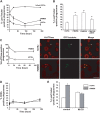
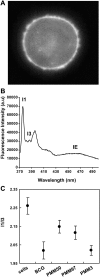
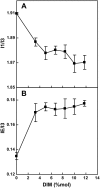
Phthiocerol dimycocerosates (DIM) are major virulence factors of Mycobacterium tuberculosis (Mtb), in particular during the early step of infection when bacilli encounter their host macrophages. However, their cellular and molecular mechanisms of action remain unknown. Using Mtb mutants deleted for genes involved in DIM biosynthesis, we demonstrated that DIM participate both in the receptor-dependent phagocytosis of Mtb and the prevention of phagosomal acidification. The effects of DIM required a state of the membrane fluidity as demonstrated by experiments conducted with cholesterol-depleting drugs that abolished the differences in phagocytosis efficiency and phagosome acidification observed between wild-type and mutant strains. The insertion of a new cholesterol-pyrene probe in living cells demonstrated that the polarity of the membrane hydrophobic core changed upon contact with Mtb whereas the lateral diffusion of cholesterol was unaffected. This effect was dependent on DIM and was consistent with the effect observed following DIM insertion in model membrane. Therefore, we propose that DIM control the invasion of macrophages by Mtb by targeting lipid organisation in the host membrane, thereby modifying its biophysical properties. The DIM-induced changes in lipid ordering favour the efficiency of receptor-mediated phagocytosis of Mtb and contribute to the control of phagosomal pH driving bacilli in a protective niche.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. - PubMed
-
- Andersen R. The chemistry of lipids of tubercle bacilli. Harvey Lect. 1940;35:271–313.
-
- Daffe M, Laneelle MA. Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria. J Gen Microbiol. 1988;134:2049–2055. - PubMed
-
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

