The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm
- PMID: 19197370
- PMCID: PMC2633037
- DOI: 10.1371/journal.pone.0004351
The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm
Abstract
Background: Concerns about false-positive HIV results led to a review of testing procedures used in a Médecins Sans Frontières (MSF) HIV programme in Bukavu, eastern Democratic Republic of Congo. In addition to the WHO HIV rapid diagnostic test algorithm (RDT) (two positive RDTs alone for HIV diagnosis) used in voluntary counselling and testing (VCT) sites we evaluated in situ a practical field-based confirmation test against western blot WB. In addition, we aimed to determine the false-positive rate of the WHO two-test algorithm compared with our adapted protocol including confirmation testing, and whether weakly reactive compared with strongly reactive rapid test results were more likely to be false positives.
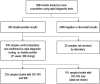
Methodology/principal findings: 2864 clients presenting to MSF VCT centres in Bukavu during January to May 2006 were tested using Determine HIV-1/2 and UniGold HIV rapid tests in parallel by nurse counsellors. Plasma samples on 229 clients confirmed as double RDT positive by laboratory retesting were further tested using both WB and the Orgenics Immunocomb Combfirm HIV confirmation test (OIC-HIV). Of these, 24 samples were negative or indeterminate by WB representing a false-positive rate of the WHO two-test algorithm of 10.5% (95%CI 6.6-15.2). 17 of the 229 samples were weakly positive on rapid testing and all were negative or indeterminate by WB. The false-positive rate fell to 3.3% (95%CI 1.3-6.7) when only strong-positive rapid test results were considered. Agreement between OIC-HIV and WB was 99.1% (95%CI 96.9-99.9%) with no false OIC-HIV positives if stringent criteria for positive OIC-HIV diagnoses were used.
Conclusions: The WHO HIV two-test diagnostic algorithm produced an unacceptably high level of false-positive diagnoses in our setting, especially if results were weakly positive. The most probable causes of the false-positive results were serological cross-reactivity or non-specific immune reactivity. Our findings show that the OIC-HIV confirmation test is practical and effective in field contexts. We propose that all double-positive HIV RDT samples should undergo further testing to confirm HIV seropositivity until the accuracy of the RDT testing algorithm has been established at programme level.
Conflict of interest statement
References
-
- Programme National de Lutte contra le VIH/SIDA et les ISTs. Rapport du passage de la surveillance sentinelle du VIH chez les femmes enceinte frequentant les services de CPN. Kinshasa, 2003–04.
-
- World Health Organization Rapid HIV Tests. 2004. Guidelines for use in HIV testing and counselling services in resource-constrained settings.
-
- Chanbancherd P, Jugsudee A, Thanomklom S, Limpairojn N, Julananto P, et al. Frequency of HIV false positivity from two sequential enzyme immunoassays in 111 639 sera. AIDS. 1999;13(15):2182–3. - PubMed
-
- Sudha T, Lakshmi V, Teja VD. Western blot profile in HIV infection. Indian J Dermatol Venereol Leprol. 2006;72(5):357–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous