MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults
- PMID: 19197383
- PMCID: PMC2634740
- DOI: 10.1371/journal.pone.0004384
MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults
Erratum in
- PLoS ONE. 2009;4(3). doi: 10.1371/annotation/5f2ae0fb-53f1-48c2-aa19-9114765ba029. Capecchi, Pamela [corrected to Capecchi, Pier Leopoldo] doi: 10.1371/annotation/5f2ae0fb-53f1-48c2-aa19-9114765ba029
Abstract
Background: Pathogenic avian influenza virus (H5N1) has the potential to cause a major global pandemic in humans. Safe and effective vaccines that induce immunologic memory and broad heterotypic response are needed.
Methods and findings: Healthy adults aged 18-60 and > 60 years (n = 313 and n = 173, respectively) were randomized (1:1) to receive two primary and one booster injection of 7.5 microg or 15 microg doses of a subunit MF59-adjuvanted H5N1 (A/Vietnam/1194/2004) (clade 1) vaccine. Safety was monitored until 6 months after booster. Immunogenicity was assessed by hemagglutination inhibition (HI), single radial hemolysis (SRH) and microneutralization assays (MN). Mild injection-site pain was the most common adverse reaction. No serious adverse events relating to the vaccine were reported. The humoral immune responses to 7.5 microg and 15 microg doses were comparable. The rates for seroprotection (HI>40; SRH>25 mm(2); MN > or = 40) after the primary vaccination ranged 72-87%. Six months after primary vaccination with the 7.5 microg dose, 18% and 21% of non-elderly and elderly adults were seroprotected; rates increased to 90% and 84%, respectively, after the booster vaccination. In the 15 microg group, seroprotection rates among non-elderly and elderly adults increased from 25% and 62% after primary vaccination to 92% and 88% after booster vaccination, respectively. A heterologous immune response to the H5N1/turkey/Turkey/05 strain was elicited after second and booster vaccinations.
Conclusions: Both formulations of MF59-adjuvanted influenza H5N1 vaccine were well tolerated. The European Union requirement for licensure for pre-pandemic vaccines was met by the lower dose tested. The presence of cross-reactive antibodies to a clade 2 heterologous strain demonstrates that this vaccine may be appropriate for pre-pandemic programs.
Trial registration: (ClinicalTrials.gov) NCT00311480.
Conflict of interest statement
Figures


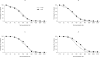
References
-
- Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis. 2002;185:1005–1010. - PubMed
-
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. - PubMed
-
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005;352:333–340. - PubMed
-
- World Health Organisation. Cumulative number of confirmed human cases of avian influenza (H5N1) reported to WHO. 2008. Geneva, WHO, Available: http://www.who.int/csr/disease/avian_influenza/country/en/. Accessed 11 August 2008.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

