Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression
- PMID: 19197388
- PMCID: PMC2634969
- DOI: 10.1371/journal.pone.0004409
Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression
Abstract
Background: Ascorbic acid (AA), or Vitamin C, is most well known as a nutritional supplement with antioxidant properties. Recently, we demonstrated that high concentrations of AA act on PMP22 gene expression and partially correct the Charcot-Marie-Tooth disease phenotype in a mouse model. This is due to the capacity of AA, but not other antioxidants, to down-modulate cAMP intracellular concentration by a competitive inhibition of the adenylate cyclase enzymatic activity. Because of the critical role of cAMP in intracellular signalling, we decided to explore the possibility that ascorbic acid could modulate the expression of other genes.
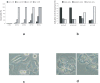
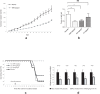
Methods and findings: Using human pangenomic microarrays, we found that AA inhibited the expression of two categories of genes necessary for cell cycle progression, tRNA synthetases and translation initiation factor subunits. In in vitro assays, we demonstrated that AA induced the S-phase arrest of proliferative normal and tumor cells. Highest concentrations of AA leaded to necrotic cell death. However, quiescent cells were not susceptible to AA toxicity, suggesting the blockage of protein synthesis was mainly detrimental in metabolically-active cells. Using animal models, we found that high concentrations of AA inhibited tumor progression in nude mice grafted with HT29 cells (derived from human colon carcinoma). Consistently, expression of tRNA synthetases and ieF2 appeared to be specifically decreased in tumors upon AA treatment.
Conclusions: AA has an antiproliferative activity, at elevated concentration that could be obtained using IV injection. This activity has been observed in vitro as well in vivo and likely results from the inhibition of expression of genes involved in protein synthesis. Implications for a clinical use in anticancer therapies will be discussed.
Conflict of interest statement
Figures



References
-
- Passage E, Norreel JC, Noack-Fraissignes P, Sanguedolce V, Pizant J, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med. 2004;10:396–401. - PubMed
-
- Kaya F, Belin S, Bourgeois P, Micaleff J, Blin O, et al. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul Disord. 2007;17:248–253. - PubMed
-
- Kaya F, Belin S, Micallef J, Blin O, Fontes M. Analysis of the benefits of vitamin cocktails in treating Charcot-Marie-Tooth disease type 1A. Muscle Nerve. 2008;38:1052–1054. - PubMed
-
- Kaya F, Belin S, Diamantidis G, Fontes M. Ascorbic acid is a regulator of the intracellular cAMP concentration: Old molecule, new functions? FEBS Lett 2008 - PubMed
-
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

