Role of neurotrophic factors in early ovarian development
- PMID: 19197802
- PMCID: PMC3525518
- DOI: 10.1055/s-0028-1108007
Role of neurotrophic factors in early ovarian development
Abstract
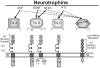
Much is known about the endocrine hormonal mechanisms controlling ovarian development. More recently, attention has focused on identifying regulatory pathways that, operating within the ovarian microenvironment, contribute to the acquisition of ovarian reproductive competence. Within this framework, the concept has developed that neurotrophins (NTs) and their Trk tyrosine kinase receptors, long thought to be exclusively required for the development of the nervous system, are also involved in the control of ovarian maturation. The ovary of several species, including rodents, sheep, cows, nonhuman primates, and humans, produce NTs and express both the high-affinity receptors and the common p75 (NTR) receptor required for signaling. Studies in humans and rodents have shown that this expression is initiated during fetal life, before the formation of primordial follicles. Gene targeting approaches have identified TrkB, the high-affinity receptor for neurotrophin-4/5 and brain-derived neurotrophic factor, as a signaling module required for follicular assembly, early follicular growth, and oocyte survival. A similar approach has shown that nerve growth factor contributes independently to the growth of primordial follicles into gonadotropin-responsive structures. Altogether, these observations indicate that NTs are important contributors to the gonadotropin-independent process underlying the formation and initiation of ovarian follicular growth.
Figures

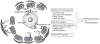
Similar articles
-
Neurotrophic and cell-cell dependent control of early follicular development.Mol Cell Endocrinol. 2000 May 25;163(1-2):67-71. doi: 10.1016/s0303-7207(99)00242-7. Mol Cell Endocrinol. 2000. PMID: 10963876 Review.
-
Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: physiological and pathophysiological implications.Hum Reprod Update. 2019 Mar 1;25(2):224-242. doi: 10.1093/humupd/dmy047. Hum Reprod Update. 2019. PMID: 30608586 Free PMC article. Review.
-
Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis.Endocrinology. 1995 Oct;136(10):4681-92. doi: 10.1210/endo.136.10.7664689. Endocrinology. 1995. PMID: 7664689
-
The importance of neuronal growth factors in the ovary.Mol Hum Reprod. 2016 Jan;22(1):3-17. doi: 10.1093/molehr/gav057. Epub 2015 Oct 20. Mol Hum Reprod. 2016. PMID: 26487421 Review.
-
Nerve growth factor is required for early follicular development in the mammalian ovary.Endocrinology. 2001 May;142(5):2078-86. doi: 10.1210/endo.142.5.8126. Endocrinology. 2001. PMID: 11316775
Cited by
-
Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Osteocalcin Gene Relationship in Energy Regulation, Bone Homeostasis and Reproductive Organs Analyzed by mRNA Quantitative Evaluation and Linear Correlation Analysis.Front Physiol. 2016 Oct 13;7:456. doi: 10.3389/fphys.2016.00456. eCollection 2016. Front Physiol. 2016. PMID: 27790153 Free PMC article.
-
Gene expression profiling reveals new potential players of gonad differentiation in the chicken embryo.PLoS One. 2011;6(9):e23959. doi: 10.1371/journal.pone.0023959. Epub 2011 Sep 9. PLoS One. 2011. PMID: 21931629 Free PMC article.
-
Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells.Int J Mol Sci. 2023 Apr 6;24(7):6837. doi: 10.3390/ijms24076837. Int J Mol Sci. 2023. PMID: 37047809 Free PMC article. Review.
-
The role of brain-derived neurotrophic factor in mouse oocyte maturation in vitro.J Huazhong Univ Sci Technolog Med Sci. 2010 Dec;30(6):781-5. doi: 10.1007/s11596-010-0658-3. Epub 2010 Dec 22. J Huazhong Univ Sci Technolog Med Sci. 2010. PMID: 21181372
-
Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure.Endocrinology. 2014 Aug;155(8):3098-111. doi: 10.1210/en.2014-1111. Epub 2014 May 30. Endocrinology. 2014. PMID: 24877631 Free PMC article.
References
-
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science. 2002;296:2178–2180. - PubMed
-
- Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab. 2002;13:169–173. - PubMed
-
- Kezel P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci. 2002;7:d1990–d1996. - PubMed
-
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005 Sep;11(5):461–471. - PubMed
-
- Kumar TR, Wang Y, Lu N, Matsuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

