Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation
- PMID: 19198003
- PMCID: PMC2693014
- DOI: 10.1002/btpr.139
Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation
Abstract
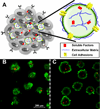
Embryonic stem cells (ESCs) are pluripotent cells capable of differentiating into all somatic and germ cell types. The intrinsic ability of pluripotent cells to generate a vast array of different cells makes ESCs a robust resource for a variety of cell transplantation and tissue engineering applications, however, efficient and controlled means of directing ESC differentiation is essential for the development of regenerative therapies. ESCs are commonly differentiated in vitro by spontaneously self-assembling in suspension culture into 3D cell aggregates called embryoid bodies (EBs), which mimic many of the hallmarks of early embryonic development, yet the 3D organization and structure of EBs also presents unique challenges to effectively direct the differentiation of the cells. ESC differentiation is strongly influenced by physical and chemical signals comprising the local extracellular microenvironment, thus current methods to engineer EB differentiation have focused primarily on spatially controlling EB size, adding soluble factors to the media, or culturing EBs on or within natural or synthetic extracellular matrices. Although most such strategies aim to influence differentiation from the exterior of EBs, engineering the microenvironment directly within EBs enables new opportunities to efficiently direct the fate of the cells by locally controlling the presentation of morphogenic cues.
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. - PubMed
-
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. - PubMed
-
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55(2):254–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources