Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis
- PMID: 19198601
- PMCID: PMC6089348
- DOI: 10.1038/ncb1833
Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis
Abstract
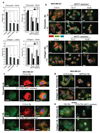
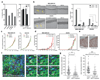
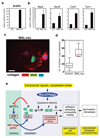
Rho GTPases control cytoskeletal dynamics through cytoplasmic effectors and regulate transcriptional activation through myocardin-related transcription factors (MRTFs), which are co-activators for serum response factor (SRF). We used RNA interference to investigate the contribution of the MRTF-SRF pathway to cytoskeletal dynamics in MDA-MB-231 breast carcinoma and B16F2 melanoma cells, in which basal MRTF-SRF activity is Rho-dependent. Depletion of MRTFs or SRF reduced cell adhesion, spreading, invasion and motility in culture, without affecting proliferation or inducing apoptosis. MRTF-depleted tumour cell xenografts showed reduced cell motility but proliferated normally. Tumour cells depleted of MRTF or SRF failed to colonize the lung from the bloodstream, being unable to persist after their arrival in the lung. Only a few genes show MRTF-dependent expression in both cell lines. Two of these, MYH9 (NMHCIIa) and MYL9 (MLC2), are also required for invasion and lung colonization. Conversely, expression of activated MAL/MRTF-A increases lung colonization by poorly metastatic B16F0 cells. Actin-based cell behaviour and experimental metastasis thus require Rho-dependent nuclear signalling through the MRTF-SRF network.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures




References
-
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–92. - PubMed
-
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. - PubMed
-
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. - PubMed
-
- Croft DR, et al. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 2004;64:8994–9001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

