Slow glycinergic transmission mediated by transmitter pooling
- PMID: 19198604
- PMCID: PMC2664096
- DOI: 10.1038/nn.2265
Slow glycinergic transmission mediated by transmitter pooling
Abstract
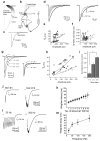
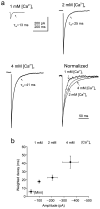
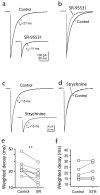
Most fast-acting neurotransmitters are rapidly cleared from synaptic regions. This feature isolates synaptic sites, rendering the time course of synaptic responses independent of the number of active synapses. We found an exception at glycinergic synapses on granule cells of the rat dorsal cochlear nucleus. Here the duration of inhibitory postsynaptic currents (IPSCs) was dependent on the number of presynaptic axons that were stimulated and on the number of vesicles that were released from each axon. Increasing the stimulus number or frequency, or blocking glycine uptake, slowed synaptic decays, whereas a low-affinity competitive antagonist of glycine receptors (GlyRs) accelerated IPSC decay. These effects could be explained by unique features of GlyRs that are activated by pooling of glycine across synapses. Functionally, increasing the number of IPSPs markedly lengthened the period of spike inhibition following the cessation of presynaptic stimulation. Thus, temporal properties of inhibition can be controlled by activity levels in multiple presynaptic cells or by adjusting release probability at individual synapses.
Figures
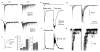



References
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
-
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

