Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis
- PMID: 19198665
- PMCID: PMC2636888
- DOI: 10.1371/journal.pone.0004417
Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis
Abstract
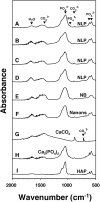
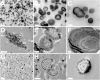
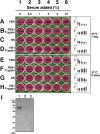
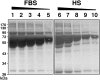
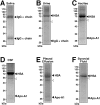
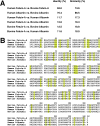
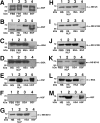
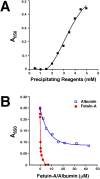
Putative living entities called nanobacteria (NB) are unusual for their small sizes (50-500 nm), pleomorphic nature, and accumulation of hydroxyapatite (HAP), and have been implicated in numerous diseases involving extraskeletal calcification. By adding precipitating ions to cell culture medium containing serum, mineral nanoparticles are generated that are morphologically and chemically identical to the so-called NB. These nanoparticles are shown here to be formed of amorphous mineral complexes containing calcium as well as other ions like carbonate, which then rapidly acquire phosphate, forming HAP. The main constituent proteins of serum-derived NB are albumin, fetuin-A, and apolipoprotein A1, but their involvement appears circumstantial since so-called NB from different body fluids harbor other proteins. Accordingly, by passage through various culture media, the protein composition of these particles can be modulated. Immunoblotting experiments reveal that antibodies deemed specific for NB react in fact with either albumin, fetuin-A, or both, indicating that previous studies using these reagents may have detected these serum proteins from the same as well as different species, with human tissue nanoparticles presumably absorbing bovine serum antigens from the culture medium. Both fetal bovine serum and human serum, used earlier by other investigators as sources of NB, paradoxically inhibit the formation of these entities, and this inhibition is trypsin-sensitive, indicating a role for proteins in this inhibitory process. Fetuin-A, and to a lesser degree albumin, inhibit nanoparticle formation, an inhibition that is overcome with time, ending with formation of the so-called NB. Together, these data demonstrate that NB are most likely formed by calcium or apatite crystallization inhibitors that are somehow overwhelmed by excess calcium or calcium phosphate found in culture medium or in body fluids, thereby becoming seeds for calcification. The structures described earlier as NB may thus represent remnants and by-products of physiological mechanisms used for calcium homeostasis, a concept which explains the vast body of NB literature as well as explains the true origin of NB as lifeless protein-mineralo entities with questionable role in pathogenesis.
Conflict of interest statement
Figures













References
-
- Kajander EO, Kuronen I, Akerman K, Pelttari A, Ciftcioglu N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth. Proc Soc Photo Opt Instrum Eng. 1997;3111:420–428.
-
- Kajander EO. Nanobacteria: propagating calcifying nanoparticles. Lett Appl Microbiol. 2006;42:549–552. - PubMed
-
- Ciftcioglu N, McKay DS, Mathew G, Kajander EO. Nanobacteria: Fact or fiction? Characteristics, detection, and medical importance of novel self-replicating, calcifying nanoparticles. J Investig Med. 2006;54:385–394. - PubMed
-
- Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO. Nanobacteria: An infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–1898. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

