Physiopathology and etiology of stone formation in the kidney and the urinary tract
- PMID: 19198886
- PMCID: PMC2839518
- DOI: 10.1007/s00467-009-1116-y
Physiopathology and etiology of stone formation in the kidney and the urinary tract
Abstract
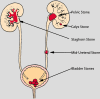
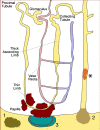
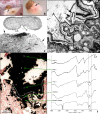
All stones share similar presenting symptoms, and urine supersaturation with respect to the mineral phase of the stone is essential for stone formation. However, recent studies using papillary biopsies of stone formers have provided a view of the histology of renal crystal deposition which suggests that the early sequence of events leading to stone formation differs greatly, depending on the type of stone and on the urine chemistry leading to supersaturation. Three general pathways for kidney stone formation are seen: (1) stones fixed to the surface of a renal papilla at sites of interstitial apatite plaque (termed Randall's plaque), as seen in idiopathic calcium oxalate stone formers; (2) stones attached to plugs protruding from the openings of ducts of Bellini, as seen in hyperoxaluria and distal tubular acidosis; and (3) stones forming in free solution in the renal collection system, as in cystinuria. The presence of hydroxyapatite crystals in either the interstitial or tubule compartment (and sometimes both) of the renal medulla in stone formers is the rule and has implications for the initial steps of stone formation and the potential for renal injury.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

