Loudness perception in the domestic cat: reaction time estimates of equal loudness contours and recruitment effects
- PMID: 19198944
- PMCID: PMC2674200
- DOI: 10.1007/s10162-009-0157-z
Loudness perception in the domestic cat: reaction time estimates of equal loudness contours and recruitment effects
Abstract
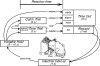
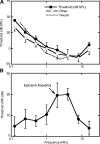
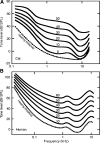
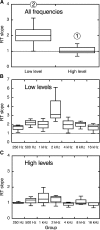
The domestic cat is the primary physiological model of loudness coding and recruitment. At present, there are no published descriptions of loudness perception in this species. This study used a reaction time task to characterize loudness perception in six behaviorally trained cats. The psychophysical approach was based on the assumption that sounds of equal loudness elicit responses of equal latency. The resulting equal latency contours reproduced well-known features of human equal loudness contours. At the completion of normal baseline measures, the cats were exposed to intense sound to investigate the behavioral correlates of loudness recruitment, the abnormally rapid growth of loudness that is commonly associated with hearing loss. Observed recruitment effects were similar in magnitude to those that have been reported in hearing-impaired humans. Linear hearing aid amplification is known to improve speech intelligibility but also exacerbate recruitment in impaired listeners. The effects of speech spectra and amplification on recruitment were explored by measuring the growth of loudness for natural and amplified vowels before and after sound exposure. Vowels produced more recruitment than tones, and the effect was exacerbated by the selective amplification of formant structure. These findings support the adequacy of the domestic cat as a model system for future investigations of the auditory processes that underlie loudness perception, recruitment, and hearing aid design.
Figures







References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1037/0096-1523.29.3.523', 'is_inner': False, 'url': 'https://doi.org/10.1037/0096-1523.29.3.523'}, {'type': 'PubMed', 'value': '12848324', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12848324/'}]}
- Arieh Y, Marks LE. Recalibrating the auditory system: a speed-accuracy analysis of intensity perception. J. Exp. Psychol. Hum. Percept. Perform. 29:523–536, 2003. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.902950106', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.902950106'}, {'type': 'PubMed', 'value': '2341636', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2341636/'}]}
- Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J. Comp. Neurol. 295:52–70, 1990. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1093/brain/8.4.512', 'is_inner': False, 'url': 'https://doi.org/10.1093/brain/8.4.512'}]}
- Cattell JM. The influence of the intensity of the stimulus on the length of the reaction time. Brain 8:512–515, 1886.
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.3406/psy.1940.5877', 'is_inner': False, 'url': 'https://doi.org/10.3406/psy.1940.5877'}]}
- Chocholle R. Variation des temps de réaction auditifs en fonction de l' intensité à diverses fréquences. L’ Année Psychologique 41:65–124, 1940.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

