Cannabinoids, endocannabinoids, and related analogs in inflammation
- PMID: 19199042
- PMCID: PMC2664885
- DOI: 10.1208/s12248-009-9084-5
Cannabinoids, endocannabinoids, and related analogs in inflammation
Abstract
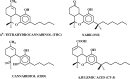
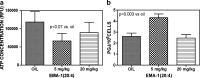
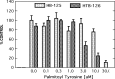
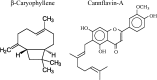
This review covers reports published in the last 5 years on the anti-inflammatory activities of all classes of cannabinoids, including phytocannabinoids such as tetrahydrocannabinol and cannabidiol, synthetic analogs such as ajulemic acid and nabilone, the endogenous cannabinoids anandamide and related compounds, namely, the elmiric acids, and finally, noncannabinoid components of Cannabis that show anti-inflammatory action. It is intended to be an update on the topic of the involvement of cannabinoids in the process of inflammation. A possible mechanism for these actions is suggested involving increased production of eicosanoids that promote the resolution of inflammation. This differentiates these cannabinoids from cyclooxygenase-2 inhibitors that suppress the synthesis of eicosanoids that promote the induction of the inflammatory process.
Figures

Similar articles
-
Cannabinoids.Curr Drug Targets CNS Neurol Disord. 2005 Oct;4(5):507-30. doi: 10.2174/156800705774322111. Curr Drug Targets CNS Neurol Disord. 2005. PMID: 16266285 Review.
-
Cannabinoids and the gut: new developments and emerging concepts.Pharmacol Ther. 2010 Apr;126(1):21-38. doi: 10.1016/j.pharmthera.2009.12.005. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117132 Review.
-
Cannabinoids, inflammation, and fibrosis.FASEB J. 2016 Nov;30(11):3682-3689. doi: 10.1096/fj.201600646R. Epub 2016 Jul 19. FASEB J. 2016. PMID: 27435265 Review.
-
Discovery of endocannabinoids and some random thoughts on their possible roles in neuroprotection and aggression.Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):93-9. doi: 10.1054/plef.2001.0340. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052029 Review.
-
Psychopharmacology of the endocannabinoids: far beyond anandamide.J Psychopharmacol. 2012 Jan;26(1):7-22. doi: 10.1177/0269881111405357. Epub 2011 Jun 7. J Psychopharmacol. 2012. PMID: 21652605 Review.
Cited by
-
Topical cannabidiol is well tolerated in individuals with a history of elite physical performance and chronic lower extremity pain.J Cannabis Res. 2023 Mar 30;5(1):11. doi: 10.1186/s42238-023-00179-8. J Cannabis Res. 2023. PMID: 36991501 Free PMC article.
-
N-Amino acid linoleoyl conjugates: anti-inflammatory activities.Bioorg Med Chem Lett. 2012 Jan 15;22(2):872-5. doi: 10.1016/j.bmcl.2011.12.040. Epub 2011 Dec 16. Bioorg Med Chem Lett. 2012. PMID: 22217875 Free PMC article.
-
Resolution of inflammation by N-arachidonoylglycine.J Cell Biochem. 2011 Nov;112(11):3227-33. doi: 10.1002/jcb.23245. J Cell Biochem. 2011. PMID: 21732409 Free PMC article.
-
Towards the use of cannabinoids as antitumour agents.Nat Rev Cancer. 2012 May 4;12(6):436-44. doi: 10.1038/nrc3247. Nat Rev Cancer. 2012. PMID: 22555283 Review.
-
Anticancer mechanisms of cannabinoids.Curr Oncol. 2016 Mar;23(2):S23-32. doi: 10.3747/co.23.3080. Epub 2016 Mar 16. Curr Oncol. 2016. PMID: 27022311 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

