Poly(ethylene glycol)-modified proteins: implications for poly(lactide-co-glycolide)-based microsphere delivery
- PMID: 19199044
- PMCID: PMC2664882
- DOI: 10.1208/s12248-009-9081-8
Poly(ethylene glycol)-modified proteins: implications for poly(lactide-co-glycolide)-based microsphere delivery
Abstract
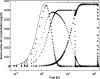
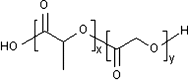
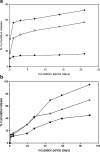
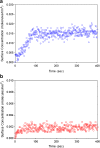
The reduced injection frequency and more nearly constant serum concentrations afforded by sustained release devices have been exploited for the chronic delivery of several therapeutic peptides via poly(lactide-co-glycolide) (PLG) microspheres. The clinical success of these formulations has motivated the exploration of similar depot systems for chronic protein delivery; however, this application has not been fully realized in practice. Problems with the delivery of unmodified proteins in PLG depot systems include high initial "burst" release and irreversible adsorption of protein to the biodegradable polymer. Further, protein activity may be lost due to the damaging effects of protein-interface and protein-surface interactions that occur during both microsphere formation and release. Several techniques are discussed in this review that may improve the performance of PLG depot delivery systems for proteins. One promising approach is the covalent attachment of poly(ethylene glycol) (PEG) to the protein prior to encapsulation in the PLG microspheres. The combination of the extended circulation time of PEGylated proteins and the shielding and potential stabilizing effects of the attached PEG may lead to improved release kinetics from PLG microsphere system and more complete release of the active conjugate.
Figures
References
-
- Harris J. M., Chess R. B. Effect of PEGylation on pharmaceuticals. Nat. Rev. 2003;2:214–221. - PubMed
-
- Hutton P., Cooper G., James F. M., James F. M., III, Butterworth J. F., IV . Anaesthesia: Fundamental principles and practice. London: Informa Health Care; 2002.
-
- Genentech, http://www.gene.com/gene/products/information/opportunistic/nutropin-aq/.... Accessed various dates.
-
- FDA, http://www.fda.gov/MEDWATCH/SAFETY/2004/oct_PI/Nutropin_PI.pdf. Accessed various dates.
-
- Pfizer, http://www.pfizer.com/files/products/uspi_somavert.pdf. Accessed various dates.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

