Designer DNA nanoarchitectures
- PMID: 19199428
- PMCID: PMC2765550
- DOI: 10.1021/bi802324w
Designer DNA nanoarchitectures
Abstract
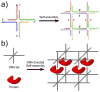
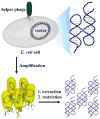
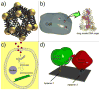
Naturally existing biological systems, from the simplest unicellular diatom to the most sophisticated organ such as the human brain, are functional self-assembled architectures. Scientists have long been dreaming about building artificial nanostructures that can mimic such elegance in nature. Structural DNA nanotechnology, which uses DNA as a blueprint and building material to organize matter with nanometer precision, represents an appealing solution to this challenge. On the basis of the knowledge of helical DNA structure and Watson-Crick base pairing rules, scientists have constructed a number of DNA nanoarchitectures with a large variety of geometries, topologies, and periodicities with considerably high yields. Modified by functional groups, those DNA nanostructures can serve as scaffolds to control the positioning of other molecular species, which opens opportunities to study intermolecular synergies, such as protein-protein interactions, as well as to build artificial multicomponent nanomachines. In this review, we summarize the principle of DNA self-assembly, describe the exciting progress of structural DNA nanotechnology in recent years, and discuss the current frontier.
Figures




References
-
- Odom TW, Huang JL, Kim P, Lieber CM. Structure and electronic properties of carbon nanotubes. J Phys Chem B. 2000;104:2794–2809.
-
- Theobald JA, Oxtoby NS, Phillips MA, Champness NR, Beton PH. Controlling molecular deposition and layer structure with supramolecular surface assemblies. Nature. 2003;424:1029–1031. - PubMed
-
- Columbo G, Soto P, Gazit E. Peptide self-assembly at the nanoscale: a challenging target for computational and experimental biology. Trends Biotechnol. 2007;25:211–218. - PubMed
-
- Seeman NC. DNA in a material world. Nature. 2003;421:427–431. - PubMed
-
- Seeman NC. Nucleic acid junctions and lattices. J Theor Biol. 1982;99:237–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

