pH dependence of a mammalian polyamine oxidase: insights into substrate specificity and the role of lysine 315
- PMID: 19199575
- PMCID: PMC2752350
- DOI: 10.1021/bi802227m
pH dependence of a mammalian polyamine oxidase: insights into substrate specificity and the role of lysine 315
Abstract
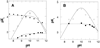
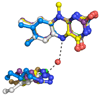
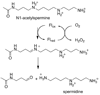
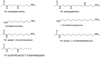
Mammalian polyamine oxidases (PAOs) catalyze the oxidation of N1-acetylspermine and N1-acetylspermidine to produce N-acetyl-3-aminopropanaldehyde and spermidine or putrescine. Structurally, PAO is a member of the monoamine oxidase family of flavoproteins. The effects of pH on the kinetic parameters of mouse PAO have been determined to provide insight into the protonation state of the polyamine required for catalysis and the roles of ionizable residues in the active site in amine oxidation. For N1-acetylspermine, N1-acetylspermidine, and spermine, the k(cat)/K(amine)-pH profiles are bell-shaped. In each case, the profile agrees with that expected if the productive form of the substrate has a single positively charged nitrogen. The pK(i)-pH profiles for a series of polyamine analogues are most consistent with the nitrogen at the site of oxidation being neutral and one other nitrogen being positively charged in the reactive form of the substrate. With N1-acetylspermine as the substrate, the value of k(red), the limiting rate constant for flavin reduction, is pH-dependent, decreasing below a pK(a) value of 7.3, again consistent with the requirement for an uncharged nitrogen for substrate oxidation. Lys315 in PAO corresponds to a conserved active site residue found throughout the monoamine oxidase family. Mutation of Lys315 to methionine has no effect on the k(cat)/K(amine) profile for spermine; the k(red) value with N1-acetylspermine is only 1.8-fold lower in the mutant protein, and the pK(a) in the k(red)-pH profile with N1-acetylspermine shifts to 7.8. These results rule out Lys315 as a source of a pK(a) in the k(cat)/K(amine) or k(cat)/k(red) profiles. They also establish that this residue does not play a critical role in amine oxidation by PAO.
Figures



Similar articles
-
Mechanistic studies of mouse polyamine oxidase with N1,N12-bisethylspermine as a substrate.Biochemistry. 2005 May 10;44(18):7079-84. doi: 10.1021/bi050347k. Biochemistry. 2005. PMID: 15865452 Free PMC article.
-
Mechanistic studies of the yeast polyamine oxidase Fms1: kinetic mechanism, substrate specificity, and pH dependence.Biochemistry. 2010 Dec 14;49(49):10440-8. doi: 10.1021/bi1016099. Epub 2010 Nov 16. Biochemistry. 2010. PMID: 21067138 Free PMC article.
-
A lysine conserved in the monoamine oxidase family is involved in oxidation of the reduced flavin in mouse polyamine oxidase.Arch Biochem Biophys. 2010 Jun 15;498(2):83-8. doi: 10.1016/j.abb.2010.04.015. Epub 2010 Apr 22. Arch Biochem Biophys. 2010. PMID: 20417173 Free PMC article.
-
Polyamine oxidase, properties and functions.Prog Brain Res. 1995;106:333-44. doi: 10.1016/s0079-6123(08)61229-7. Prog Brain Res. 1995. PMID: 8584670 Review.
-
Interconversion, catabolism and elimination of the polyamines.Med Biol. 1981 Dec;59(5-6):334-46. Med Biol. 1981. PMID: 7040832 Review.
Cited by
-
Mechanistic studies of the role of a conserved histidine in a mammalian polyamine oxidase.Arch Biochem Biophys. 2012 Dec 1;528(1):45-9. doi: 10.1016/j.abb.2012.08.007. Epub 2012 Aug 30. Arch Biochem Biophys. 2012. PMID: 22959971 Free PMC article.
-
Mechanistic studies of para-substituted N,N'-dibenzyl-1,4-diaminobutanes as substrates for a mammalian polyamine oxidase.Biochemistry. 2009 Dec 29;48(51):12305-13. doi: 10.1021/bi901694s. Biochemistry. 2009. PMID: 19911805 Free PMC article.
-
Structure of the flavoprotein tryptophan 2-monooxygenase, a key enzyme in the formation of galls in plants.Biochemistry. 2013 Apr 16;52(15):2620-6. doi: 10.1021/bi4001563. Epub 2013 Apr 4. Biochemistry. 2013. PMID: 23521653 Free PMC article.
-
KDM1 class flavin-dependent protein lysine demethylases.Biopolymers. 2015 Jul;104(4):213-46. doi: 10.1002/bip.22643. Biopolymers. 2015. PMID: 25787087 Free PMC article. Review.
-
Mechanism of Flavoprotein l-6-Hydroxynicotine Oxidase: pH and Solvent Isotope Effects and Identification of Key Active Site Residues.Biochemistry. 2017 Feb 14;56(6):869-875. doi: 10.1021/acs.biochem.6b01160. Epub 2017 Jan 26. Biochemistry. 2017. PMID: 28080034 Free PMC article.
References
-
- Tabor CW, Tabor H. Polyamines. Ann. Rev. Biochem. 1984;53:749–790. - PubMed
-
- Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Ann. Rev. Pharmacol. Toxicol. 1995;35:55–91. - PubMed
-
- Lin PKT, Dance AM, Bestwick C, Milne L. The biological activities of new polyamine derivatives as potential therapeutic agents. Biochem. Soc. Trans. 2003;31:407–410. - PubMed
-
- Wang Y, Casero RA., Jr Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J. Biochem. (Tokyo) 2006;139:17–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

