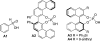Highly enantioselective hydrogenation of enamides catalyzed by chiral phosphoric acids
- PMID: 19199770
- PMCID: PMC2765520
- DOI: 10.1021/ol802860u
Highly enantioselective hydrogenation of enamides catalyzed by chiral phosphoric acids
Abstract
A highly enantioselective hydrogenation of enamides catalyzed by a dual chiral-achiral acid system was developed. By employing a substoichiometric amount of a chiral phosphoric acid and acetic acid, catalyst loadings as low as 1 mol % of the chiral catalyst were sufficient to provide excellent yield and enantioselectivity of the reduction product.
Figures
Similar articles
-
Highly enantioselective Rh-catalyzed hydrogenation of beta,gamma-unsaturated phosphonates with chiral ferrocene-based monophosphoramidite ligands.J Org Chem. 2009 Dec 4;74(23):9191-4. doi: 10.1021/jo901619c. J Org Chem. 2009. PMID: 19877610
-
Brønsted acid-catalyzed, highly enantioselective addition of enamides to in situ-generated ortho-quinone methides: a domino approach to complex acetamidotetrahydroxanthenes.Chemistry. 2015 Feb 2;21(6):2348-52. doi: 10.1002/chem.201406044. Epub 2014 Dec 8. Chemistry. 2015. PMID: 25488376
-
Cooperative catalysis: combining an achiral metal catalyst with a chiral Brønsted acid enables highly enantioselective hydrogenation of imines.Chemistry. 2013 Oct 11;19(42):14187-93. doi: 10.1002/chem.201302437. Epub 2013 Sep 9. Chemistry. 2013. PMID: 24019056
-
Recent Progress in Asymmetric Relay Catalysis of Metal Complex with Chiral Phosphoric Acid.Top Curr Chem (Cham). 2019 Dec 27;378(1):9. doi: 10.1007/s41061-019-0263-2. Top Curr Chem (Cham). 2019. PMID: 31879793 Review.
-
Chiral Brønsted acids for asymmetric organocatalysis.Top Curr Chem. 2010;291:395-456. doi: 10.1007/978-3-642-02815-1_1. Top Curr Chem. 2010. PMID: 21494945 Review.
Cited by
-
What is the role of acid-acid interactions in asymmetric phosphoric acid organocatalysis? A detailed mechanistic study using interlocked and non-interlocked catalysts.Chem Sci. 2020 Apr 7;11(17):4381-4390. doi: 10.1039/d0sc01026j. Chem Sci. 2020. PMID: 34122895 Free PMC article.
-
Chiral Phosphoric Acid Catalyzed Conversion of Epoxides into Thiiranes: Mechanism, Stereochemical Model, and New Catalyst Design.Angew Chem Int Ed Engl. 2022 Feb 21;61(9):e202113204. doi: 10.1002/anie.202113204. Epub 2022 Jan 14. Angew Chem Int Ed Engl. 2022. PMID: 34889494 Free PMC article.
-
Divergent Stereoselectivity in Phosphothreonine (pThr)-Catalyzed Reductive Aminations of 3-Amidocyclohexanones.J Org Chem. 2018 Apr 20;83(8):4491-4504. doi: 10.1021/acs.joc.8b00207. Epub 2018 Apr 5. J Org Chem. 2018. PMID: 29547285 Free PMC article.
-
General, Modular Access toward Immobilized Chiral Phosphoric Acid Catalysts and Their Application in Flow Chemistry.ACS Catal. 2024 Mar 29;14(8):5550-5559. doi: 10.1021/acscatal.4c00985. eCollection 2024 Apr 19. ACS Catal. 2024. PMID: 38660609 Free PMC article.
-
Single-operation deracemization of 3H-indolines and tetrahydroquinolines enabled by phase separation.J Am Chem Soc. 2013 Sep 25;135(38):14090-3. doi: 10.1021/ja4082827. Epub 2013 Sep 11. J Am Chem Soc. 2013. PMID: 24025122 Free PMC article.
References
-
-
For reviews, see: Ouellet G, Walji AM, Macmillan DWC. Acc. Chem. Res. 2007;40:1327.You S-L. Chemistry, an Asian journal. 2007;2:820.Connon SJ. Org. Biomol. Chem. 2007;5:3407..
-
-
-
For reviews with considerable chiral phosphoric acid coverage see: Akiyama T, Itoh J, Fuchibe K. Adv. Synth. Catal. 2006;348:999.Connon SJ. Angew. Chem., Int. Ed. 2006;45:3909.Akiyama T. Chem. Rev. 2007;107:5744.Terada M. Chem. Commun. 2008:4097. For recent reports see: Rueping M, Nachtsheim BJ, Moreth SA, Bolte M. Angew. Chem., Int. Ed. 2008;47:593.Wang X, List B. Angew. Chem., Int. Ed. 2008;47:1119.Akiyama T, Honma Y, Itoh J, Fuchibe K. Adv. Synth. Catal. 2008;350:399.Jiao P, Nakashima D, Yamamoto H. Angew. Chem. Int. Ed. 2008;47:2411.Jiang J, Yu J, Sun X-X, Rao Q-Q, Gong L-Z. Angew. Chem., Int. Ed. 2008;47:2458.Xu S, Wang Z, Zhang X, Zhang X, Ding K. Angew. Chem., Int. Ed. 2008;47:2840.Chen X-H, Zhang W-Q, Gong L-Z. J. Am. Chem. Soc. 2008;130:5652.Enders D, Narine AA, Toulgoat F, Bisschops T. Angew. Chem., Int. Ed. 2008;47:5661.Zhang G-W, Wang L, Nie J, Ma J-A. Adv. Synth. Catal. 2008;350:1457.Cheon CH, Yamamoto H. J. Am. Chem. Soc. 2008;130:9246.Hu W, Xu X, Zhou J, Liu W-J, Huang H, Hu J, Yang L, Gong L-Z. J. Am. Chem. Soc. 2008;130:7782.Rueping M, Antonchick AP. Org. Lett. 2008;10:1731.Giera D, Sickert M, Schneider C. Org. Lett. 2008;10:4259.Cheng X, Goddard R, Buth G, List B. Angew. Chem., Int. Ed. 2008;47:5079.Sickert Marcel, Schneider Christoph. Angew. Chem., Int. Ed. 2008;47:3631.Itoh J, Fuchibe K, Akiyama T. Angew. Chem., Int. Ed. 2008;47:4016.Terada M, Soga K, Momiyama N. Angew. Chem., Int. Ed. 2008;47:4122.Rueping M, Antonchick AP. Angew. Chem., Int. Ed. 2008;47:5836.Rueping M, Theissmann T, Kuenkel A, Koenigs RM. Angew. Chem., Int. Ed. 2008;47:6798.Kang Q, Zheng X-J, You S-L. Chem. Eur. J. 2008;14:3539.Cheng X, Vellalath S, Goddard R, List B. J. Am. Chem. Soc. 2008;130:15786.Rueping M, Antonchick AP. Angew. Chem., Int. Ed. 2008;47:10090..
-
-
-
For chiral phosphoric acid catalyzed reduction reactions, see: Rueping M, Sugiono E, Azap C, Theissmann T, Bolte M. Org. Lett. 2005;7:3781.Hoffmann S, Seayad AM, List B. Angew. Chem., Int. Ed. 2005;44:7424.Storer RI, Carrera DE, Ni Y, MacMillan DWC. J. Am. Chem. Soc. 2006;128:84.Martin NJA, List B. J. Am. Chem. Soc. 2006;128:13368.Hoffmann S, Nicoletti M, List B. J. Am. Chem. Soc. 2006;128:13074.Rueping M, Antonchick AP, Theissmann T. Angew. Chem., Int. Ed. 2006;45:3683.Mayer S, List B. Angew. Chem., Int. Ed. 2006;45:4193.Rueping M, Antonchick AP, Theissmann T. Angew. Chem., Int. Ed. 2006;45:6751.Zhou J, List B. J. Am. Chem. Soc. 2007;129:7498.Rueping M, Antonchick AP. Angew. Chem., Int. Ed. 2007;46:4562.Guo Q-S, Du D-M, Xu J. Angew. Chem., Int. Ed. 2008;47:759.Rueping M, Theissmann T, Raja S, Bats JW. Adv. Synth. Catal. 2008;350:1001.Kang Q, Zhao Z–A, You S-L. Adv. Synth. Catal. 2007;349:1657.Kang Q, Zhao Z-A, You S-L. Org. Lett. 2008;10:2031.Martin NJA, Cheng X, List B. J. Am. Chem. Soc. 2008;130:13862..
-
-
- Li G, Liang Y, Antilla JC. J. Am. Chem. Soc. 2007;129:5830. - PubMed
- Rowland GB, Zhang H, Rowland EB, Chennamadhavuni S, Wang Y, Antilla JC. J. Am. Chem. Soc. 2005;127:15696. - PubMed
- Rowland GB, Rowland EB, Liang Y, Antilla JC. Org. Lett. 2007;9:2609. - PubMed
- Li G, Rowland GB, Rowland EB, Antilla JC. Org. Lett. 2007;9:4065. - PubMed
- Liang Y, Rowland EB, Rowland GB, Perman JA, Antilla JC. Chem. Commun. 2007;43:4477. - PubMed
- Rowland EB, Rowland GB, Rivera-Otero E, Antilla JC. J. Am. Chem. Soc. 2007;129:12084. - PubMed
- Li G, Fronczek FR, Antilla JC. J. Am. Chem. Soc. 2008;130:12216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources