Context-dependent substrate recognition by protein farnesyltransferase
- PMID: 19199818
- PMCID: PMC2765569
- DOI: 10.1021/bi801710g
Context-dependent substrate recognition by protein farnesyltransferase
Abstract
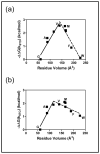
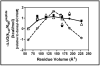
Prenylation is a posttranslational modification whereby C-terminal lipidation leads to protein localization to membranes. A C-terminal "Ca(1)a(2)X" sequence has been proposed as the recognition motif for two prenylation enzymes, protein farnesyltransferase (FTase) and protein geranylgeranyltransferase type I. To define the parameters involved in recognition of the a(2) residue, we performed structure-activity analysis which indicates that FTase discriminates between peptide substrates based on both the hydrophobicity and steric volume of the side chain at the a(2) position. For nonpolar side chains, the dependence of the reactivity on side chain volume at this position forms a pyramidal pattern with a maximal activity near the steric volume of valine. This discrimination occurs at a step in the kinetic mechanism that is at or before the farnesylation step. Furthermore, a(2) selectivity is also affected by the identity of the adjacent X residue, leading to context-dependent substrate recognition. Context-dependent a(2) selectivity suggests that FTase recognizes the sequence downstream of the conserved cysteine as a set of two or three cooperative, interconnected recognition elements as opposed to three independent amino acids. These findings expand the pool of proposed FTase substrates in cells. A better understanding of the molecular recognition of substrates performed by FTase will aid in both designing new FTase inhibitors as therapeutic agents and characterizing proteins involved in prenylation-dependent cellular pathways.
Figures






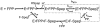
References
-
- Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Ann Rev Biochem. 1996;65:241–269. - PubMed
-
- Benetka W, Koranda M, Eisenhaber F. Protein prenylation: An (almost) comprehensive overview on discovery history, enzymology, and significance in physiology and disease. Monatsh Chem. 2006;137:1241–1281.
-
- Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. - PubMed
-
- Casey PJ. Lipid modifications of g proteins. Curr Opin Cell Biol. 1994;6:219–225. - PubMed
-
- Marshall CJ. Protein prenylation: A mediator of protein-protein interactions. Science. 1993;259:219–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

