Effects of herpes simplex virus amplicon transduction on murine dendritic cells
- PMID: 19199821
- PMCID: PMC2828628
- DOI: 10.1089/hum.2008.160
Effects of herpes simplex virus amplicon transduction on murine dendritic cells
Abstract
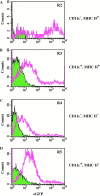
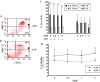
The herpes simplex virus (HSV)-based amplicon is a versatile vaccine platform that has been preclinically vetted as a gene-based immunotherapeutic for cancer, HIV, and neurodegenerative disorders. Although it is well known that injection of dendritic cells (DCs) transduced ex vivo with helper virus-free HSV amplicon vectors expressing disease-relevant antigens induces antigen-specific immune responses, the cellular receptor(s) by which the amplicon virion gains entry into DCs, as well as the effects that viral vector transduction impinges on the physiological status of these cells, is less understood. Herein, we examine the effects of amplicon transduction on mouse bone marrow-derived DCs. We demonstrate that HSV-1 cellular receptors HveC and HveA are expressed on the cell surface of murine DCs, and that HSV amplicons transduce DCs at high efficiency (>90%) with minimal effects on cell viability. Transduction of dendritic cells with amplicons induces a transient DC maturation phenotype as represented by self-limited upregulation of MHCII and CD11c markers. Mature DCs are less sensitive to HSV amplicon transduction than immature DCs regarding DC-related surface marker maintenance. From this and our previous work, we conclude that HSV amplicons transduce DCs efficiently, but impart differential and transient physiological effects on mature and immature DC pools, which will facilitate fine-tuning of this vaccination platform and further exploit its potential in immunotherapy.
Figures




Similar articles
-
Murine Bone Marrow-Derived Dendritic Cells Transduced by Light-Helper-Dependent Herpes Simplex Virus-1 Amplicon Vector Acquire a Mature Dendritic Cell Phenotype.Hum Gene Ther Methods. 2015 Jun;26(3):93-102. doi: 10.1089/hgtb.2015.042. Hum Gene Ther Methods. 2015. PMID: 26046494
-
Dendritic cells transduced with HSV-1 amplicons expressing prostate-specific antigen generate antitumor immunity in mice.Hum Gene Ther. 2001 Oct 10;12(15):1867-79. doi: 10.1089/104303401753153929. Hum Gene Ther. 2001. PMID: 11589829
-
Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry.J Virol. 1998 Sep;72(9):7064-74. doi: 10.1128/JVI.72.9.7064-7074.1998. J Virol. 1998. PMID: 9696799 Free PMC article.
-
The interaction between dendritic cells and herpes simplex virus-1.Curr Top Microbiol Immunol. 2003;276:145-61. doi: 10.1007/978-3-662-06508-2_7. Curr Top Microbiol Immunol. 2003. PMID: 12797447 Review.
-
Amplicons as vaccine vectors.Curr Gene Ther. 2006 Jun;6(3):383-92. doi: 10.2174/156652306777592009. Curr Gene Ther. 2006. PMID: 16787189 Review.
Cited by
-
Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice.J Immunol. 2011 Apr 1;186(7):3927-33. doi: 10.4049/jimmunol.1003735. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357536 Free PMC article.
-
Herpes simplex virus and varicella zoster virus, the house guests who never leave.Herpesviridae. 2012 Jun 12;3(1):5. doi: 10.1186/2042-4280-3-5. Herpesviridae. 2012. PMID: 22691604 Free PMC article.
References
-
- Alnaeeli M. Park J. Mahamed D. Penninger J.M. Teng Y.T. Dendritic cells at the osteo-immune interface: Implications for inflammation-induced bone loss. J. Bone Miner. Res. 2007;22:775–780. - PubMed
-
- Bennett J.J. Delman K.A. Burt B.M. Mariotti A. Malhotra S. Zager J. Petrowsky H. Mastorides S. Federoff H. Fong Y. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9:935–945. - PubMed
-
- Bowers W.J. Howard D.F. Federoff H.J. Discordance between expression and genome transfer titering of HSV amplicon vectors: Recommendation for standardized enumeration. Mol. Ther. 2000;1:294–299. - PubMed
-
- Bowers W.J. Howard D.F. Brooks A.I. Halterman M.W. Federoff H.J. Expression of vhs and VP16 during HSV-1 helper virus-free amplicon packaging enhances titers. Gene Ther. 2001;8:111–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

