SUMOylation participates in induction of ischemic tolerance
- PMID: 19200349
- PMCID: PMC2692380
- DOI: 10.1111/j.1471-4159.2009.05957.x
SUMOylation participates in induction of ischemic tolerance
Abstract
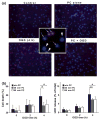
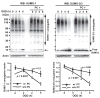
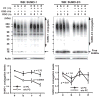
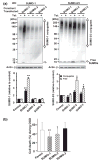
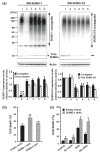
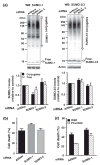
Ground squirrels in hibernation torpor have been shown to have striking increases in global SUMOylation on tissue immunoblots. Here, we find evidence that global SUMOylation is also involved in ischemic tolerance in primary cortical neuronal cultures (from rats and mice) and SHSY5Y human neuroblastoma cells. Cultured cortical neurons preconditioned by sublethal oxygen/glucose deprivation (OGD) were less vulnerable to severe OGD than non-preconditioned neurons. Preconditioned neurons maintained elevated SUMO-1 conjugation levels (and, to a lesser extent those of SUMO-2/3) on western blots in contrast to non-preconditioned cells. Further, cortical neurons and SHSY5Y cells in which transfected SUMO-1 or SUMO-2 were over-expressed showed increased survival after severe OGD. In contrast, cell cultures subjected to depletion of endogenous SUMO-1 protein by RNAi had reduced survival after exposure to this form of in vitro ischemia and an attenuated protective response to preconditioning. These findings suggest that maintenance of a globally elevated SUMO-1 (and maybe SUMO-2/3) conjugation level as revealed by immunoblot assays is a component of ischemic tolerance.
Figures

References
-
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. - PubMed
-
- Chinnadurai G. Transcriptional regulation by C-terminal binding proteins. Int J Biochem Cell Biol. 2007;39:1593–1607. - PubMed
-
- Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–289. - PubMed
-
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

