Sex differences in the genetic architecture of lifespan in a seed beetle: extreme inbreeding extends male lifespan
- PMID: 19200350
- PMCID: PMC2657122
- DOI: 10.1186/1471-2148-9-33
Sex differences in the genetic architecture of lifespan in a seed beetle: extreme inbreeding extends male lifespan
Abstract
Background: Sex differences in lifespan are ubiquitous throughout the animal kingdom but the causes underlying this phenomenon remain poorly understood. Several explanations based on asymmetrical inheritance patterns (sex chromosomes or mitochondrial DNA) have been proposed, but these ideas have rarely been tested experimentally. Alternatively, sexual dimorphism in lifespan could result from sex-specific selection, caused by fundamental differences in how males and females optimize their fitness by allocating resources into current and future reproduction.
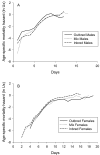
Results: Here we used sex-specific responses to inbreeding to study the genetic architecture of lifespan and mortality rates in Callosobruchus maculatus, a seed beetle that shows sexual dimorphism in lifespan. Two independent assays revealed opposing sex-specific responses to inbreeding. The combined data set showed that inbred males live longer than outbred males, while females show the opposite pattern. Both sexes suffered reduced fitness measured as lifetime reproductive success as a result of inbreeding.
Conclusion: No model based on asymmetrical inheritance can explain increased male lifespan in response to inbreeding. Our results are however compatible with models based on sex-specific selection on reproductive strategies. We therefore suggest that sex-specific differences in lifespan in this species primarily result from sexually divergent selection.
Figures


References
-
- Williams GC. Pleiotropy, Natural-Selection, and the Evolution of Senescence. Evolution. 1957;11:398–411. doi: 10.2307/2406060. - DOI
-
- Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Chicago: Aldine; 1972. pp. 136–179.
-
- Trivers R. Social evolution. Menlo Park, Calif.: Benjamin/Cummings Pub. Co; 1985.
-
- Liker A, Szekely T. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution. 2005;59:890–897. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

