Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids
- PMID: 19200454
- PMCID: PMC2761210
- DOI: 10.1016/j.freeradbiomed.2008.11.021
Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids
Abstract
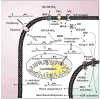
The signaling mediators nitric oxide ( NO) and oxidized lipids, once viewed to transduce metabolic and inflammatory information via discrete and independent pathways, are now appreciated as interdependent regulators of immune response and metabolic homeostasis. The interactions between these two classes of mediators result in reciprocal control of mediator synthesis that is strongly influenced by the local chemical environment. The relationship between the two pathways extends beyond coregulation of NO and eicosanoid formation to converge via the nitration of unsaturated fatty acids to yield nitro derivatives (NO(2)-FA). These pluripotent signaling molecules are generated in vivo as an adaptive response to oxidative inflammatory conditions and manifest predominantly anti-inflammatory signaling reactions. These actions of NO(2)-FA are diverse, with these species serving as a potential chemical reserve of NO, reacting with cellular nucleophiles to posttranslationally modify protein structure, function, and localization. In this regard these species act as potent endogenous ligands for peroxisome proliferator-activated receptor gamma. Functional consequences of these signaling mechanisms have been shown in multiple model systems, including the inhibition of platelet and neutrophil functions, induction of heme oxygenase-1, inhibition of LPS-induced cytokine release in monocytes, increased insulin sensitivity and glucose uptake in adipocytes, and relaxation of preconstricted rat aortic segments. These observations have propelled further in vitro and in vivo studies of mechanisms of NO(2)-FA signaling and metabolism, highlighting the therapeutic potential of this class of molecules as anti-inflammatory drug candidates.
Figures
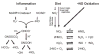



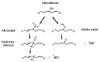

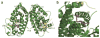
References
-
- Nathan C. Nitric oxide as a secretory product of mammalian cells. Federation of American Societies for Experimental Biology Journal. 1992;6:3051–3064. - PubMed
-
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–680. - PubMed
-
- Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22(12):477–481. - PubMed
-
- Lancaster JR, Jr, Ignarro LJ. Nitric Oxide - Biology and Pathobiology. Academic Press; San Diego: 2002. The Physical Properties of Nitric Oxide.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

