Should individual PI3 kinase isoforms be targeted in cancer?
- PMID: 19200708
- PMCID: PMC3142565
- DOI: 10.1016/j.ceb.2008.12.007
Should individual PI3 kinase isoforms be targeted in cancer?
Abstract
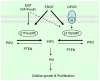
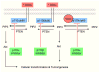
Activation of the phosphoinositide-3-kinase (PI3K) signaling pathway is frequently found in common human cancers, brought about by oncogenic receptor tyrosine kinases (RTKs) acting upstream, PTEN loss, or activating mutations of PI3K itself. Recent studies have delineated distinct but overlapping functions in cell signaling and tumorigenesis for p110alpha and p110beta, the two major catalytic subunits of PI3K expressed in the tissues of origin for the common tumor types. In most cell types studied, p110alpha carries the majority of the PI3K signal in classic RTK signal transduction, while p110beta responds to GPCRs. Both p110alpha and p110beta function in cellular transformation induced by alterations in components of PI3K pathway. Specifically, p110alpha is essential for the signaling and growth of tumors driven by PIK3CA mutations and/or oncogenic RTKs/Ras, whereas p110beta is the major isoform in mediating PTEN-deficient tumorigenesis. While pan-PI3K inhibitors are currently being tested in the clinic, p110 isoform-specific inhibition holds promise as a therapeutic strategy.
Figures

References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. - PubMed
-
- Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. - PubMed
-
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. - PubMed
-
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

