Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning
- PMID: 19200729
- PMCID: PMC3654375
- DOI: 10.1016/j.tcb.2009.01.001
Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning
Abstract
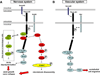
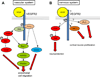
The nervous and vascular systems are both exquisitely branched and complicated systems and their proper development requires careful guidance of nerves and vessels. The recent realization that common ligand-receptor pairs are used in guiding the patterning of both systems has prompted the question of whether similar signaling pathways are used in both systems. This review highlights recent progress in our understanding of the similarities and differences in the intracellular signaling mechanisms downstream of semaphorins, ephrins and vascular endothelial growth factor in neurons and endothelial cells during neural and vascular development. We present evidence that similar intracellular signaling principles underlying cytoskeletal regulation are used to control neural and vascular guidance, although the specific molecules used in neurons and endothelial cells are often different.
Figures



Similar articles
-
Guidance of vascular and neural network formation.Curr Opin Neurobiol. 2005 Feb;15(1):108-15. doi: 10.1016/j.conb.2005.01.008. Curr Opin Neurobiol. 2005. PMID: 15721752 Review.
-
Neuronal clues to vascular guidance.Exp Cell Res. 2006 Mar 10;312(5):668-75. doi: 10.1016/j.yexcr.2005.11.009. Epub 2005 Dec 5. Exp Cell Res. 2006. PMID: 16330027 Review.
-
Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development.Genes Dev. 1999 May 1;13(9):1055-66. doi: 10.1101/gad.13.9.1055. Genes Dev. 1999. PMID: 10323857 Review. No abstract available.
-
Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues.Cell Mol Life Sci. 2013 May;70(10):1685-703. doi: 10.1007/s00018-013-1278-4. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475066 Free PMC article. Review.
-
Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels.Circ Res. 2012 Jan 6;110(1):34-46. doi: 10.1161/CIRCRESAHA.111.249847. Epub 2011 Nov 10. Circ Res. 2012. PMID: 22076636 Free PMC article.
Cited by
-
Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer.Cell Res. 2012 Jan;22(1):23-32. doi: 10.1038/cr.2011.198. Epub 2011 Dec 13. Cell Res. 2012. PMID: 22157652 Free PMC article. Review.
-
Semaphorins and their Signaling Mechanisms.Methods Mol Biol. 2017;1493:1-25. doi: 10.1007/978-1-4939-6448-2_1. Methods Mol Biol. 2017. PMID: 27787839 Free PMC article. Review.
-
Neurovascular coupling in the developing neonatal brain at rest.Hum Brain Mapp. 2020 Feb 1;41(2):503-519. doi: 10.1002/hbm.24818. Epub 2019 Oct 10. Hum Brain Mapp. 2020. PMID: 31600024 Free PMC article.
-
The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary.Dev Cell. 2011 Oct 18;21(4):642-54. doi: 10.1016/j.devcel.2011.09.004. Dev Cell. 2011. PMID: 22014522 Free PMC article.
-
Diverse roles for axon guidance pathways in adult tissue architecture and function.Nat Sci (Weinh). 2022 Oct;2(4):e20220021. doi: 10.1002/ntls.20220021. Epub 2022 Oct 2. Nat Sci (Weinh). 2022. PMID: 37456985 Free PMC article.
References
-
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. - PubMed
-
- Freitas C, Larrivee B, Eichmann A. Netrins and UNC5 receptors in angiogenesis. Angiogenesis. 2008;11(1):23–29. - PubMed
-
- Legg JA, et al. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11(1):13–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

