Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells
- PMID: 19200802
- PMCID: PMC2670853
- DOI: 10.1016/j.stem.2008.11.015
Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells
Abstract
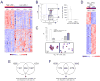
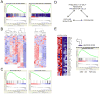
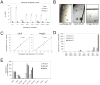
The genetic programs that promote retention of self-renewing leukemia stem cells (LSCs) at the apex of cellular hierarchies in acute myeloid leukemia (AML) are not known. In a mouse model of human AML, LSCs exhibit variable frequencies that correlate with the initiating MLL oncogene and are maintained in a self-renewing state by a transcriptional subprogram more akin to that of embryonic stem cells (ESCs) than to that of adult stem cells. The transcription/chromatin regulatory factors Myb, Hmgb3, and Cbx5 are critical components of the program and suffice for Hoxa/Meis-independent immortalization of myeloid progenitors when coexpressed, establishing the cooperative and essential role of an ESC-like LSC maintenance program ancillary to the leukemia-initiating MLL/Hox/Meis program. Enriched expression of LSC maintenance and ESC-like program genes in normal myeloid progenitors and poor-prognosis human malignancies links the frequency of aberrantly self-renewing progenitor-like cancer stem cells (CSCs) to prognosis in human cancer.
Figures
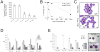


Comment in
-
The MLLgnant consequences of reverting to an embryonic transcriptional program.Cell Stem Cell. 2009 Feb 6;4(2):97-8. doi: 10.1016/j.stem.2009.01.008. Cell Stem Cell. 2009. PMID: 19200795
References
-
- Adams JM, Kelly PN, Dakic A, Nutt SL, Strasser A. Response to Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. - PubMed
-
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

