A two-step mechanism for stem cell activation during hair regeneration
- PMID: 19200804
- PMCID: PMC2668200
- DOI: 10.1016/j.stem.2008.12.009
A two-step mechanism for stem cell activation during hair regeneration
Erratum in
- Cell Stem Cell. 2009 May 8;4(5):464
Abstract
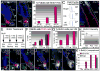
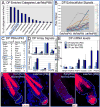
Hair follicles (HFs) undergo cyclic bouts of degeneration, rest, and regeneration. During rest (telogen), the hair germ (HG) appears as a small cell cluster between the slow-cycling bulge and dermal papilla (DP). Here we show that HG cells are derived from bulge stem cells (SCs) but become responsive quicker to DP-promoting signals. In vitro, HG cells also proliferate sooner but display shorter-lived potential than bulge cells. Molecularly, they more closely resemble activated bulge rather than transit-amplifying (matrix) cells. Transcriptional profiling reveals precocious activity of both HG and DP in late telogen, accompanied by Wnt signaling in HG and elevated FGFs and BMP inhibitors in DP. FGFs and BMP inhibitors participate with Wnts in exerting selective and potent stimuli to the HG both in vivo and in vitro. Our findings suggest a model where HG cells fuel initial steps in hair regeneration, while the bulge is the engine maintaining the process.
Figures





Comment in
-
The river of stem cells.Cell Stem Cell. 2009 Feb 6;4(2):100-2. doi: 10.1016/j.stem.2009.01.003. Cell Stem Cell. 2009. PMID: 19200797 Free PMC article.
References
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
-
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205–2214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

