An improved in vitro and in vivo Sindbis virus expression system through host and virus engineering
- PMID: 19200810
- PMCID: PMC2730471
- DOI: 10.1016/j.virusres.2008.12.019
An improved in vitro and in vivo Sindbis virus expression system through host and virus engineering
Abstract
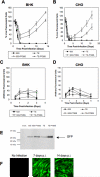
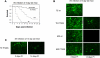
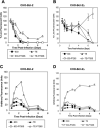
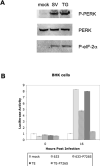
The Sindbis viral expression system enables the rapid production of high levels of recombinant protein in mammalian cells; however, this expression is typically limited to transient production due to the cytotoxicity of the virus. Limiting the lethality inherent in the Sindbis virus vector in order to enable long term, sustained expression of recombinant proteins may be possible. In this study, modifications to virus and host have been combined in order to reduce the cytopathic effects. Non-cytopathic replication competent viruses of two Sindbis viral strains, TE and 633, were developed using a non-structural protein (nsP) P726S point mutation in order to obtain persistent heterologous gene expression in infected Baby Hamster Kidney (BHK) cells and Chinese Hamster Ovary (CHO) cells. Cells infected with the P726S variant viruses were able to recover after infection, while cells infected with normal virus died within 3 days. The P726S mutation did not reduce the susceptibility of 5- and 14-day-old mice to 633 and TE viruses in vivo. In addition, animal survival with the P726S variant viruses was increased and GFP expression was sustained for at least 14 days while the 633 and TE infection resulted in short-term GFP expression or an earlier mortality. Modifications to the host BHK and CHO cells themselves were subsequently undertaken by including the anti-apoptotic gene Bcl-2 and a deletion mutant of Bcl-2 (Bcl-2Delta) as another method for limiting the cytopathic effects of the Sindbis virus. The inclusion of anti-apoptotic genes permitted higher production of heterologous GFP protein following Sindbis virus infection, and the combination of the TE-P726S virus and the CHO-Bcl-2Delta cell line showed the greatest improvement in cell survival. Sindbis virus infection also induced ER stress in mammalian cells as detected by increased PERK phosphorylation and ATF4 translation. Overexpression of Parkin, an E3 ubiquitin ligase that can protect cells against agents that induce ER stress, suppressed Sindbis virus-induced cell death in both BHK cells and in vivo studies in mice. Such findings show that viral and host modifications can improve cell survival and production of heterologous proteins, change viral behavior in vitro and in vivo, and assist in the development of new expression or gene delivery vehicles.
Figures


Similar articles
-
Production of Noncapped Genomic RNAs Is Critical to Sindbis Virus Disease and Pathogenicity.mBio. 2020 Dec 1;11(6):e02675-20. doi: 10.1128/mBio.02675-20. mBio. 2020. PMID: 33262258 Free PMC article.
-
A comparison of the properties of a Bcl-xL variant to the wild-type anti-apoptosis inhibitor in mammalian cell cultures.Metab Eng. 2003 Oct;5(4):230-45. doi: 10.1016/s1096-7176(03)00044-2. Metab Eng. 2003. PMID: 14642351
-
Aven and Bcl-xL enhance protection against apoptosis for mammalian cells exposed to various culture conditions.Biotechnol Bioeng. 2004 Mar 20;85(6):589-600. doi: 10.1002/bit.10913. Biotechnol Bioeng. 2004. PMID: 14966800
-
Neuronal apoptosis pathways in Sindbis virus encephalitis.Prog Mol Subcell Biol. 2004;36:71-93. doi: 10.1007/978-3-540-74264-7_5. Prog Mol Subcell Biol. 2004. PMID: 15171608 Review.
-
A review of alphavirus replication in neurons.Neurosci Biobehav Rev. 1998 Oct;22(6):721-3. doi: 10.1016/s0149-7634(98)00010-4. Neurosci Biobehav Rev. 1998. PMID: 9809307 Review.
Cited by
-
Screening and large-scale expression of membrane proteins in mammalian cells for structural studies.Nat Protoc. 2014 Nov;9(11):2574-85. doi: 10.1038/nprot.2014.173. Epub 2014 Oct 9. Nat Protoc. 2014. PMID: 25299155 Free PMC article.
-
Recombinant Vaccinia virus-coded interferon inhibitor B18R: Expression, refolding and a use in a mammalian expression system with a RNA-vector.PLoS One. 2017 Dec 7;12(12):e0189308. doi: 10.1371/journal.pone.0189308. eCollection 2017. PLoS One. 2017. PMID: 29216299 Free PMC article.
-
Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response.PLoS Pathog. 2012;8(7):e1002783. doi: 10.1371/journal.ppat.1002783. Epub 2012 Jul 5. PLoS Pathog. 2012. PMID: 22792064 Free PMC article.
-
Recombinant protein vaccines produced in insect cells.Vaccine. 2012 Feb 27;30(10):1759-66. doi: 10.1016/j.vaccine.2012.01.016. Epub 2012 Jan 17. Vaccine. 2012. PMID: 22265860 Free PMC article. Review.
-
The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis.Crit Rev Microbiol. 2015 Jun;41(2):150-64. doi: 10.3109/1040841X.2013.813899. Epub 2013 Sep 9. Crit Rev Microbiol. 2015. PMID: 25168431 Free PMC article. Review.
References
-
- Boorsma M, Saudan P, Pfruender H, Bailey JE, Schlesinger S, Renner WA, Bachmann MF. Alphavirus cDNA-based expression vectors: effects of RNA transcription and nuclear export. Biotechnol Bioeng. 2003;81(5):553–62. - PubMed
-
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22(53):8608–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

