Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation
- PMID: 19201385
- PMCID: PMC2803112
- DOI: 10.1016/j.vaccine.2009.01.088
Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation
Abstract
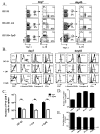
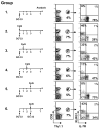
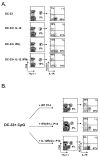
Adjuvants are commonly used in vaccines to augment immune response, but how the inflammatory cytokines elicited by adjuvants directly influence effector and memory CD8 T cell differentiation remains poorly characterized. Here, we used a peptide-pulsed dendritic cell (DC) vaccination model to examine the role of primary cytokines, IL-12 and IFNgamma, elicited by CpG-B adjuvant on CD8 T cell priming and memory CD8 T cell development. During DC vaccination, simultaneous exposure to antigen and a heterologous Listeria infection, CpG-B or IL-12 enhanced a portion of the effector CD8 T cells to expand and differentiate to a larger extent. Simultaneously, this also decreased their ability to become long-lived memory CD8 T cells. However, development of memory CD8 T cells and their precursors was largely unaffected by the additional inflammatory cytokines. Moreover, IL-12 production by the antigen-presenting cell (APC) was not required during DC+CpG vaccination or Listeria infection, but rather 'bystander' macrophages and DCs appeared to be the physiologically relevant cellular sources of this cytokine. Furthermore, IFNgamma induced by CpG was required in vivo for optimal production of IL-12, which in turn, influenced effector CD8 T cell longevity. Together, these findings demonstrate the importance of an interconnected multicellular network between APCs, naïve T cells and bystander cells of the innate immune system that regulate effector and memory CD8 T cell development during vaccination.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
Detailed analysis for inducing specific CD8 T cells via a CpG-DNA adjuvant.Expert Rev Vaccines. 2009 Jun;8(6):699-703. doi: 10.1586/erv.09.36. Expert Rev Vaccines. 2009. PMID: 19485751
References
-
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006 Feb 24;124(4):849–63. - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature reviews. 2002 Apr;2(4):251–62. - PubMed
-
- Letvin NL. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity. 2007 Sep;27(3):366–9. - PubMed
-
- Schwarz K, Meijerink E, Speiser DE, Tissot AC, Cielens I, Renhof R, et al. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. European journal of immunology. 2005 Mar;35(3):816–21. - PubMed
-
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nature medicine. 2005 Apr;11(4 Suppl):S63–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

